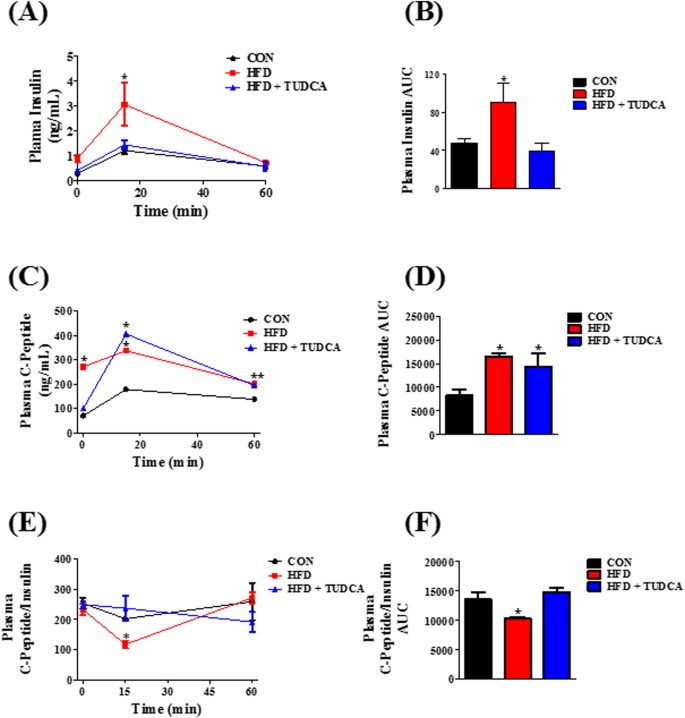
A recent study published in Scientific Reports has shed light on the potential benefits of the bile acid TUDCA (tauroursodeoxycholic acid) in improving insulin clearance and liver health in obese mice. The research highlights the role of TUDCA in increasing the expression of insulin-degrading enzyme (IDE) in the liver, which is crucial for maintaining insulin levels and glucose metabolism.
Key Findings
- Insulin Clearance: TUDCA treatment led to enhanced insulin clearance in obese mice, which is essential for preventing hyperinsulinemia, a condition characterized by excess insulin in the blood and associated with obesity and type 2 diabetes.
- Liver Health: The liver plays a pivotal role in insulin degradation. The study found that TUDCA increases the expression of IDE in the liver, suggesting a protective effect against liver diseases often linked to obesity.
- Insulin Resistance: Obesity is a major risk factor for developing insulin resistance. The study indicates that TUDCA may help improve insulin sensitivity, thereby reducing the risk of type 2 diabetes and metabolic syndrome.
- Cellular Mechanisms: The research delves into the cellular pathways involved, such as the PI3K/Akt pathway, which is significant for various cellular processes including metabolism and cell survival.
Source: Nature’s.
Implications for Human Health
While the study was conducted on animal models, the findings offer promising insights into potential treatments for obesity-related conditions in humans. TUDCA’s ability to modulate insulin levels and liver function could pave the way for new therapeutic strategies.
Conclusion
The study concludes that TUDCA is a potential therapeutic agent for enhancing insulin clearance and improving liver health in the context of obesity. Further research is needed to explore its efficacy and safety in human subjects.
For more detailed information, read the full article on Nature’s website.