Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment (PDQ®): Treatment – Patient Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
Childhood acute myeloid leukemia (AML) is a type of cancer in which the bone marrow makes a large number of abnormal blood cells.
Childhood acute myeloid leukemia (AML) is a cancer of the blood and bone marrow. AML is also called acute myelogenous leukemia, acute myeloblastic leukemia, acute granulocytic leukemia, and acute nonlymphocytic leukemia. Cancers that are acute usually get worse quickly if they are not treated. Cancers that are chronic usually get worse slowly.
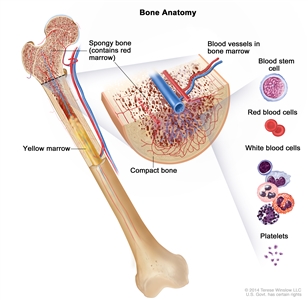
Anatomy of the bone. The bone is made up of compact bone, spongy bone, and bone marrow. Compact bone makes up the outer layer of the bone. Spongy bone is found mostly at the ends of bones and contains red marrow. Bone marrow is found in the center of most bones and has many blood vessels. There are two types of bone marrow: red and yellow. Red marrow contains blood stem cells that can become red blood cells, white blood cells, or platelets. Yellow marrow is made mostly of fat.
Leukemia and other diseases of the blood and bone marrow may affect red blood cells, white blood cells, and platelets.
Normally, the bone marrow makes blood stem cells (immature cells) that become mature blood cells over time. A blood stem cell may become a myeloid stem cell or a lymphoid stem cell. A lymphoid stem cell becomes a white blood cell.
A myeloid stem cell becomes one of three types of mature blood cells:
- Red blood cells that carry oxygen and other substances to all tissues of the body.
- White blood cells that fight infection and disease.
- Platelets that form blood clots to stop bleeding.
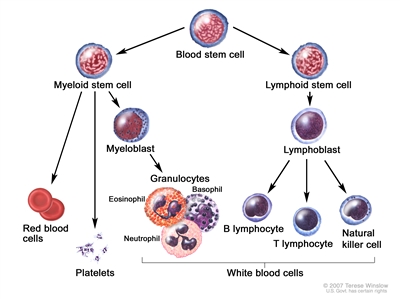
Blood cell development. A blood stem cell goes through several steps to become a red blood cell, platelet, or white blood cell.
In AML, the myeloid stem cells usually become a type of immature white blood cell called myeloblasts (or myeloid blasts). The myeloblasts, or leukemia cells, in AML are abnormal and do not become healthy white blood cells. The leukemia cells can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. When this happens, infection, anemia, or easy bleeding may occur.
The leukemia cells can spread outside the blood to other parts of the body, including the central nervous system (brain and spinal cord), skin, and gums. Sometimes leukemia cells form a solid tumor called a granulocytic sarcoma or chloroma.
Other myeloid diseases can affect the blood and bone marrow.
Transient abnormal myelopoiesis (TAM)
TAM is a disorder of the bone marrow that can develop in newborns who have Down syndrome. It usually goes away on its own within the first 3 months of life. Infants who have TAM have an increased chance of developing AML before the age of 3 years. TAM is also called transient myeloproliferative disorder or transient leukemia.
Acute promyelocytic leukemia (APL)
APL is a subtype of AML. In APL, some genes on chromosome 15 switch places with some genes on chromosome 17 and an abnormal gene called PML-RARA is made. The PML-RARA gene sends a message that stops promyelocytes (a type of white blood cell) from maturing. The promyelocytes (leukemia cells) can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. Problems with severe bleeding and blood clots may also occur. This is a serious health problem that needs treatment as soon as possible.
Juvenile myelomonocytic leukemia (JMML)
JMML is a rare childhood cancer that is most common in children around the age of 2 years and is more common in boys. In JMML, too many myeloid blood stem cells become myelocytes and monocytes (two types of white blood cells). Some of these myeloid blood stem cells never become mature white blood cells. These immature cells, called blasts, are unable to do their usual work. Over time, the myelocytes, monocytes, and blasts crowd out the red blood cells and platelets in the bone marrow. When this happens, infection, anemia, or easy bleeding may occur.
Chronic myelogenous leukemia (CML)
CML often begins in an early myeloid blood cell when a certain gene change occurs. A section of genes, that includes the ABL gene, on chromosome 9 changes place with a section of genes on chromosome 22, which has the BCR gene. This makes a very short chromosome 22 (called the Philadelphia chromosome) and a very long chromosome 9. An abnormal BCR-ABL gene is formed on chromosome 22. The BCR-ABL gene tells the blood cells to make too much of a protein called tyrosine kinase. Tyrosine kinase causes too many white blood cells (leukemia cells) to be made in the bone marrow. The leukemia cells can build up in the blood and bone marrow so there is less room for healthy white blood cells, red blood cells, and platelets. When this happens, infection, anemia, or easy bleeding may occur. CML is rare in children.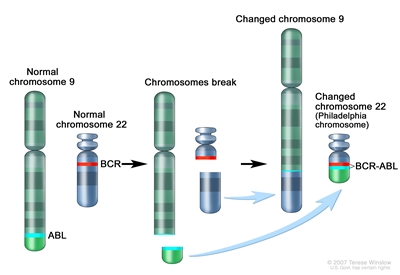
Philadelphia chromosome. A piece of chromosome 9 and a piece of chromosome 22 break off and trade places. The BCR-ABL gene is formed on chromosome 22 where the piece of chromosome 9 attaches. The changed chromosome 22 is called the Philadelphia chromosome.
Myelodysplastic syndromes (MDS)
MDS occur less often in children than in adults. In MDS, the bone marrow makes too few red blood cells, white blood cells, and platelets. These blood cells may not mature and enter the blood. The type of MDS depends on the type of blood cell that is affected.
The treatment for MDS depends on how low the numbers of red blood cells, white blood cells, or platelets are. Over time, MDS may become AML.
This summary is about childhood AML, TAM, childhood APL, JMML, childhood CML, and childhood MDS. See the Childhood Acute Lymphoblastic Leukemia Treatment summary for information about the treatment of childhood acute lymphoblastic leukemia.
AML or MDS may occur after treatment with certain chemotherapy drugs and/or radiation therapy.
Cancer treatment with certain chemotherapy drugs and/or radiation therapy may cause therapy-related AML (t-AML) or therapy -related MDS (t-MDS). The risk of these therapy-related myeloid diseases depends on the total dose of the chemotherapy drugs used and the radiation dose and treatment field. Some patients also have an inherited risk for t-AML and t-MDS. These therapy-related diseases usually occur within 7 years after treatment, but are rare in children.
The risk factors for childhood AML, APL, JMML, CML, and MDS are similar.
Anything that increases your risk of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn’t mean that you will not get cancer. Talk with your child’s doctor if you think your child may be at risk. These and other factors may increase the risk of childhood AML, APL, JMML, CML, and MDS:
- Having a brother or sister, especially a twin, with leukemia.
- Being Hispanic.
- Being exposed to cigarette smoke or alcohol before birth.
- Having a personal history of aplastic anemia.
- Having a personal or family history of MDS.
- Having a family history of AML.
- Past treatment with chemotherapy or radiation therapy.
- Being exposed to ionizing radiation or chemicals such as benzene.
- Having certain syndromes or inherited disorders, such as:
- Down syndrome.
- Aplastic anemia.
- Fanconi anemia.
- Neurofibromatosis type 1.
- Noonan syndrome.
- Shwachman-Diamond syndrome.
- Li-Fraumeni syndrome.
Signs and symptoms of childhood AML, APL, JMML, CML, or MDS include fever, feeling tired, and easy bleeding or bruising.
These and other signs and symptoms may be caused by childhood AML, APL, JMML, CML, or MDS or by other conditions. Check with a doctor if your child has any of the following:
- Fever with or without an infection.
- Night sweats.
- Shortness of breath.
- Weakness or feeling tired.
- Easy bruising or bleeding.
- Petechiae (flat, pinpoint spots under the skin caused by bleeding).
- Pain in the bones or joints.
- Pain or feeling of fullness below the ribs.
- Painless lumps in the neck, underarm, stomach, groin, or other parts of the body. In childhood AML, these lumps, called leukemia cutis, may be blue or purple.
- Painless lumps that are sometimes around the eyes. These lumps, called chloromas, are sometimes seen in childhood AML and may be blue-green.
- An eczema -like skin rash.
The signs and symptoms of TAM may include the following:
- Swelling all over the body.
- Shortness of breath.
- Trouble breathing.
- Weakness or feeling tired.
- Bleeding a lot, even from a small cut.
- Petechiae (flat, pinpoint spots under the skin caused by bleeding).
- Pain below the ribs.
- Skin rash.
- Jaundice (yellowing of the skin and whites of the eyes).
- Headache, trouble seeing, and confusion.
Sometimes TAM does not cause any symptoms at all and is diagnosed after a routine blood test.
Tests that examine the blood and bone marrow are used to detect (find) and diagnose childhood AML, TAM, APL, JMML, CML, and MDS.
The following tests and procedures may be used:
- Physical exam and history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
- Complete blood count (CBC) with differential: A procedure in which a sample of blood is drawn and checked for the following:
- The number of red blood cells and platelets.
- The number and type of white blood cells.
- The amount of hemoglobin (the protein that carries oxygen) in the red blood cells.
- The portion of the blood sample made up of red blood cells.
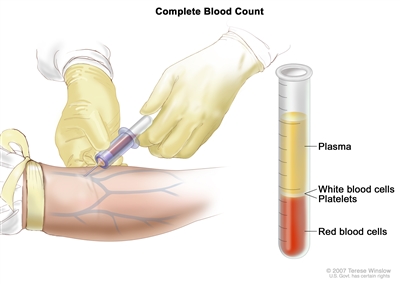
Complete blood count (CBC). Blood is collected by inserting a needle into a vein and allowing the blood to flow into a tube. The blood sample is sent to the laboratory and the red blood cells, white blood cells, and platelets are counted. The CBC is used to test for, diagnose, and monitor many different conditions. - Blood chemistry studies: A procedure in which a blood sample is checked to measure the amounts of certain substances released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
- Chest x-ray: An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- Biopsy: The removal of cells or tissues so they can be viewed under a microscope by a pathologist to check for signs of cancer. Biopsies that may be done include the following:
- Bone marrow aspiration and biopsy: The removal of bone marrow, blood, and a small piece of bone by inserting a hollow needle into the hipbone or breastbone.
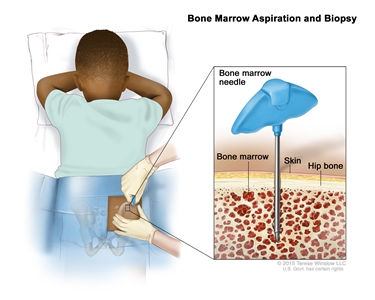
Bone marrow aspiration and biopsy. After a small area of skin is numbed, a bone marrow needle is inserted into the child’s hip bone. Samples of blood, bone, and bone marrow are removed for examination under a microscope. - Tumor biopsy: A biopsy of a chloroma may be done.
- Lymph node biopsy: The removal of all or part of a lymph node.
- Bone marrow aspiration and biopsy: The removal of bone marrow, blood, and a small piece of bone by inserting a hollow needle into the hipbone or breastbone.
- Immunophenotyping: A process used to identify cells, based on the types of antigens or markers on the surface of the cell, that may include special staining of the blood and bone marrow cells. This process is used to diagnose the subtype of AML by comparing the cancer cells to normal cells of the immune system.
- Cytogenetic analysis: A laboratory test in which cells in a sample of blood or bone marrow are viewed under a microscope to look for certain changes in the chromosomes. Changes in the chromosomes include when part of one chromosome is switched with part of another chromosome, part of one chromosome is missing or repeated, or part of one chromosome is turned upside down.
The following test is a type of cytogenetic analysis:
- FISH (fluorescence in situ hybridization): A laboratory technique used to look at genes or chromosomes in cells and tissues. Pieces of DNA that contain a fluorescent dye are made in the laboratory and added to cells or tissues on a glass slide. When these pieces of DNA bind to specific genes or areas of chromosomes on the slide, they light up when viewed under a microscope with a special light.
- Molecular testing: A laboratory test to check for certain genes, proteins, or other molecules in a sample of blood or bone marrow. Molecular tests also check for certain changes in a gene or chromosome that may cause or affect the chance of developing AML. A molecular test may be used to help plan treatment, find out how well treatment is working, or make a prognosis.
- Lumbar puncture: A procedure used to collect a sample of cerebrospinal fluid (CSF) from the spinal column. This is done by placing a needle between two bones in the spine and into the CSF around the spinal cord and removing a sample of the fluid. The sample of CSF is checked under a microscope for signs that leukemia cells have spread to the brain and spinal cord. This procedure is also called an LP or spinal tap.
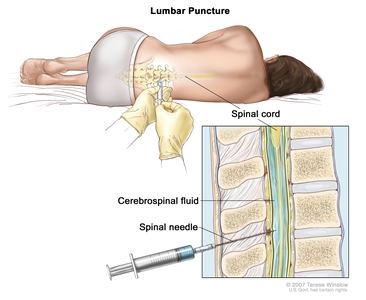
Lumbar puncture. A patient lies in a curled position on a table. After a small area on the lower back is numbed, a spinal needle (a long, thin needle) is inserted into the lower part of the spinal column to remove cerebrospinal fluid (CSF, shown in blue). The fluid may be sent to a laboratory for testing.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis (chance of recovery) and treatment options for childhood AML depend on the following:
- The age of the child when the cancer is diagnosed.
- The race or ethnic group of the child.
- Whether the child is greatly overweight.
- Number of white blood cells in the blood at diagnosis.
- Whether the AML occurred after previous cancer treatment.
- The subtype of AML.
- Whether there are certain chromosome or gene changes in the leukemia cells.
- Whether the child has Down syndrome. Most children with AML and Down syndrome can be cured of their leukemia.
- Whether the leukemia is in the central nervous system (brain and spinal cord).
- How quickly the leukemia responds to treatment.
- Whether the AML is newly diagnosed (untreated) or has recurred after treatment.
- The length of time since treatment ended, for AML that has recurred.
The prognosis for childhood APL depends on the following:
- Number of white blood cells in the blood at diagnosis.
- Whether there are certain chromosome or gene changes in the leukemia cells.
- Whether the APL is newly diagnosed (untreated) or has recurred after treatment.
The prognosis and treatment options for JMML depend on the following:
- The age of the child when the cancer is diagnosed.
- The type of gene affected and the number of genes that have changes.
- How many monocytes (a type of white blood cell) are in the blood.
- How much hemoglobin is in the blood.
- Whether the JMML is newly diagnosed (untreated) or has recurred after treatment.
The prognosis and treatment options for childhood CML depend on the following:
- How long it has been since the patient was diagnosed.
- How many blast cells are in the blood.
- Whether and how fully the blast cells disappear from the blood and bone marrow after therapy has started.
- Whether the CML is newly diagnosed (untreated) or has recurred after treatment.
The prognosis and treatment options for MDS depend on the following:
- Whether the MDS was caused by previous cancer treatment.
- How low the numbers of red blood cells, white blood cells, or platelets are.
- Whether the MDS is newly diagnosed (untreated) or has recurred after treatment.
Stages of Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
Once childhood acute myeloid leukemia (AML) has been diagnosed, tests are done to find out if the cancer has spread to other parts of the body.
The following tests and procedures may be used to determine if the leukemia has spread from the blood to other parts of the body:
- Lumbar puncture: A procedure used to collect a sample of cerebrospinal fluid (CSF) from the spinal column. This is done by placing a needle between two bones in the spine and into the CSF around the spinal cord and removing a sample of the fluid. The sample of CSF is checked under a microscope for signs that leukemia cells have spread to the brain and spinal cord. This procedure is also called an LP or spinal tap.
- Biopsy of the testicles, ovaries, or skin: The removal of cells or tissues from the testicles, ovaries, or skin so they can be viewed under a microscope to check for signs of cancer. This is done only if something unusual about the testicles, ovaries, or skin is found during the physical exam.
There is no standard staging system for childhood AML, childhood acute promyelocytic leukemia (APL), juvenile myelomonocytic leukemia (JMML), childhood chronic myelogenous leukemia (CML), or myelodysplastic syndromes (MDS).
The extent or spread of cancer is usually described as stages. Instead of stages, treatment of childhood AML, childhood APL, JMML, childhood CML, and MDS is based on one or more of the following:
- The type of disease or the subtype of AML.
- Whether leukemia has spread outside the blood and bone marrow.
- Whether the disease is newly diagnosed, in remission, or recurrent.
Newly diagnosed childhood AML
Newly diagnosed childhood AML has not been treated except to relieve signs and symptoms such as fever, bleeding, or pain, and one of the following is found:
- More than 20% of the cells in the bone marrow are blasts (leukemia cells).
or
- Less than 20% of the cells in the bone marrow are blasts and there is a certain change in the chromosome.
Childhood AML in remission
In childhood AML in remission, the disease has been treated and the following are found:
- The complete blood count is almost normal.
- Less than 5% of the cells in the bone marrow are blasts (leukemia cells).
- There are no signs or symptoms of leukemia in the brain, spinal cord, or other parts of the body.
Recurrent childhood AML has come back after it has been treated.
In recurrent childhood AML, the cancer may come back in the blood and bone marrow or in other parts of the body, such as the central nervous system (brain and spinal cord).
In refractory childhood AML, the cancer does not respond to treatment.
Treatment Option Overview
There are different types of treatment for children with AML, TAM, APL, JMML, CML, and MDS.
Different types of treatment are available for children with acute myeloid leukemia (AML), transient abnormal myelopoiesis (TAM), acute promyelocytic leukemia (APL), juvenile myelomonocytic leukemia (JMML), chronic myelogenous leukemia (CML), and myelodysplastic syndromes (MDS). Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment.
Because AML and other myeloid disorders are rare in children, taking part in a clinical trial should be considered. Some clinical trials are open only to patients who have not yet started treatment.
Treatment is planned by a team of health care providers who are experts in treating childhood leukemia and other diseases of the blood.
Treatment will be overseen by a pediatric oncologist, a doctor who specializes in treating children with cancer. The pediatric oncologist works with other healthcare providers who are experts in treating children with leukemia and who specialize in certain areas of medicine. These may include the following specialists:
- Pediatrician.
- Hematologist.
- Medical oncologist.
- Pediatric surgeon.
- Radiation oncologist.
- Neurologist.
- Neuropathologist.
- Neuroradiologist.
- Pediatric nurse specialist.
- Social worker.
- Rehabilitation specialist.
- Psychologist.
Treatment for childhood acute myeloid leukemia may cause side effects.
For information about side effects that begin during treatment for cancer, see our Side Effects page.
Regular follow-up exams are very important. Side effects from cancer treatment that begin after treatment and continue for months or years are called late effects. Late effects of cancer treatment may include the following:
- Physical problems.
- Changes in mood, feelings, thinking, learning, or memory.
- Second cancers (new types of cancer).
Some late effects may be treated or controlled. It is important that parents of children who are treated for AML or other blood diseases talk with their child’s doctors about the effects cancer treatment can have on their child. (See the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information).
The treatment of childhood AML usually has two phases.
The treatment of childhood AML is done in phases:
- Induction therapy: This is the first phase of treatment. The goal is to kill the leukemia cells in the blood and bone marrow. This puts the leukemia into remission.
- Consolidation /intensification therapy: This is the second phase of treatment. It begins once the leukemia is in remission. The goal of therapy is to kill any remaining leukemia cells that are hiding and may not be active but could begin to regrow and cause a relapse.
Treatment called central nervous system (CNS) prophylaxis therapy may be given during the induction phase of therapy. Because standard doses of chemotherapy may not reach leukemia cells in the CNS (brain and spinal cord), the leukemia cells are able to hide in the CNS. Intrathecal chemotherapy is able to reach leukemia cells in the CNS. It is given to kill the leukemia cells and lessen the chance the leukemia will recur (come back).
Seven types of standard treatment are used for childhood AML, TAM, childhood APL, JMML, childhood CML, and MDS.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). When chemotherapy is placed directly into the cerebrospinal fluid (intrathecal chemotherapy), an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy). Combination chemotherapy is treatment using more than one chemotherapy drug.
The way the chemotherapy is given depends on the type of cancer being treated. In AML, chemotherapy given by mouth, vein, or into the cerebrospinal fluid is used.
In AML, the leukemia cells may spread to the brain and/or spinal cord. Chemotherapy given by mouth or vein to treat AML may not cross the blood-brain barrier to get into the fluid that surrounds the brain and spinal cord. Instead, chemotherapy is injected into the fluid-filled space to kill leukemia cells that may have spread there (intrathecal chemotherapy).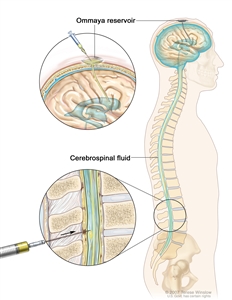
Intrathecal chemotherapy. Anticancer drugs are injected into the intrathecal space, which is the space that holds the cerebrospinal fluid (CSF, shown in blue). There are two different ways to do this. One way, shown in the top part of the figure, is to inject the drugs into an Ommaya reservoir (a dome-shaped container that is placed under the scalp during surgery; it holds the drugs as they flow through a small tube into the brain). The other way, shown in the bottom part of the figure, is to inject the drugs directly into the CSF in the lower part of the spinal column, after a small area on the lower back is numbed.
See Drugs Approved for Acute Myeloid Leukemia for more information.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
- External radiation therapy uses a machine outside the body to send radiation toward the cancer.
- Internal radiation therapy uses a radioactive substance sealed in needles, seeds, wires, or catheters that are placed directly into or near the cancer.
The way the radiation therapy is given depends on the type of the cancer being treated. In childhood AML, external radiation therapy may be used to treat a chloroma that does not respond to chemotherapy.
Stem cell transplant
Chemotherapy is given to kill cancer cells or other abnormal blood cells. Healthy cells, including blood-forming cells, are also destroyed by the cancer treatment. Stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient or a donor and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body’s blood cells.
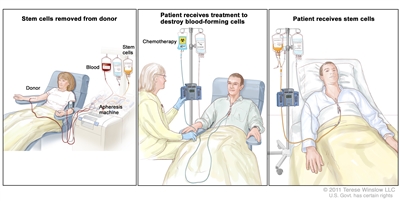
Stem cell transplant. (Step 1): Blood is taken from a vein in the arm of the donor. The patient or another person may be the donor. The blood flows through a machine that removes the stem cells. Then the blood is returned to the donor through a vein in the other arm. (Step 2): The patient receives chemotherapy to kill blood-forming cells. The patient may receive radiation therapy (not shown). (Step 3): The patient receives stem cells through a catheter placed into a blood vessel in the chest.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells without harming normal cells. Types of targeted therapy include the following:
- Tyrosine kinase inhibitor therapy: Tyrosine kinase inhibitor (TKI) therapy blocks signals needed for tumors to grow. TKIs block the enzyme (tyrosine kinase) that causes stem cells to become more white blood cells (blasts) than the body needs. TKIs may be used with chemotherapy drugs as adjuvant therapy (treatment given after the initial treatment, to lower the risk that the cancer will come back).
- Imatinib, dasatinib, and nilotinib are types of TKIs that are used to treat childhood CML.
- Sorafenib and trametinib are being studied in the treatment of childhood leukemia.
- Monoclonal antibody therapy: Monoclonal antibody therapy uses antibodies made in the laboratory, from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attach to the substances and kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells.
- Gemtuzumab is a type of monoclonal antibody that is attached to a chemotherapy drug. It is used in the treatment of AML.
Selinexor is a targeted therapy drug that is being studied to treat refractory or recurrent childhood AML.
See Drugs Approved for Leukemia for more information.
Other drug therapy
Lenalidomide may be used to lessen the need for transfusions in patients who have myelodysplastic syndromes caused by a specific chromosome change. It is also being studied in the treatment of children with recurrent and refractory AML.
Arsenic trioxide and all-trans retinoic acid (ATRA) are drugs that kill certain types of leukemia cells, stop the leukemia cells from dividing, or help the leukemia cells mature into white blood cells. These drugs are used in the treatment of acute promyelocytic leukemia.
See Drugs Approved for Acute Myeloid Leukemia for more information.
Watchful waiting
Watchful waiting is closely monitoring a patient’s condition without giving any treatment until signs or symptoms appear or change. It is sometimes used to treat MDS or transient abnormal myelopoiesis (TAM).
Supportive care
Supportive care is given to lessen the problems caused by the disease or its treatment. All patients with leukemia receive supportive care treatments. Supportive care may include the following:
- Transfusion therapy: A way of giving red blood cells, white blood cells, or platelets to replace blood cells destroyed by disease or cancer treatment. The blood may be donated from another person or it may have been taken from the patient earlier and stored until needed.
- Drug therapy, such as antibiotics or antifungal agents.
- Leukapheresis: A procedure in which a special machine is used to remove white blood cells from the blood. Blood is taken from the patient and put through a blood cell separator where the white blood cells are removed. The rest of the blood is then returned to the patient’s bloodstream. Leukapheresis is used to treat patients with very high white blood cell counts.
New types of treatment are being tested in clinical trials.
This summary section describes treatments that are being studied in clinical trials. It may not mention every new treatment being studied. Information about clinical trials is available from the NCI website.
Immunotherapy
Immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or biological therapy.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today’s standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer or to find out the stage of the cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your child’s condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.
Treatment Options for Childhood Acute Myeloid Leukemia
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of newly diagnosed childhood acute myeloid leukemia (AML) during the induction phase may include the following:
- Combination chemotherapy.
- Targeted therapy with a monoclonal antibody (gemtuzumab).
- Central nervous system prophylaxis therapy with intrathecal chemotherapy.
- Radiation therapy, for patients with a granulocytic sarcoma (chloroma) if chemotherapy does not work.
- Stem cell transplant, for patients with therapy-related AML.
Treatment of childhood AML during the remission phase (consolidation /intensification therapy) depends on the subtype of AML and may include the following:
- Combination chemotherapy.
- High-dose chemotherapy followed by stem cell transplant using blood stem cells from a donor.
Treatment of refractory childhood AML may include the following:
- Lenalidomide therapy.
- A clinical trial of chemotherapy and targeted therapy (selinexor).
- A new combination chemotherapy regimen.
Treatment of recurrent childhood AML may include the following:
- Combination chemotherapy.
- Combination chemotherapy and stem cell transplant, for patients who have had a second complete remission.
- A clinical trial of chemotherapy and targeted therapy (selinexor).
Treatment Options for Transient Abnormal Myelopoiesis or Children with Down Syndrome and AML
For information about the treatments listed below, see the Treatment Option Overview section.
Transient abnormal myelopoiesis (TAM) usually goes away on its own. For TAM that does not go away on its own or causes other health problems, treatment may include the following:
- Supportive care, including transfusion therapy or leukapheresis.
- Chemotherapy.
Treatment of acute myeloid leukemia (AML) in children aged 4 years or younger who have Down syndrome may include the following:
- Combination chemotherapy plus central nervous system prophylaxis therapy with intrathecal chemotherapy.
- A clinical trial of a new chemotherapy regimen that depends on how the child responds to initial chemotherapy.
Treatment of AML in children older than 4 years who have Down syndrome may be the same as treatment for children without Down syndrome.
Treatment Options for Childhood Acute Promyelocytic Leukemia
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of newly diagnosed childhood acute promyelocytic leukemia (APL) may include the following:
- All-trans retinoic acid (ATRA) plus chemotherapy.
- Arsenic trioxide therapy.
- A clinical trial of ATRA and arsenic trioxide therapy with or without chemotherapy.
Treatment of childhood APL during the remission phase (consolidation /intensification therapy) may include the following:
- All-trans retinoic acid (ATRA) plus chemotherapy.
Treatment of recurrent childhood APL may include the following:
- Arsenic trioxide therapy.
- All-trans retinoic acid therapy (ATRA) plus chemotherapy.
- Targeted therapy with a monoclonal antibody (gemtuzumab).
- Stem cell transplant using blood stem cells from the patient or a donor.
Treatment Options for Juvenile Myelomonocytic Leukemia
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of juvenile myelomonocytic leukemia (JMML) may include the following:
- Combination chemotherapy followed by stem cell transplant. If JMML recurs after stem cell transplant, a second stem cell transplant may be done.
Treatment of refractory or recurrent childhood JMML may include the following:
- A clinical trial of targeted therapy with a tyrosine kinase inhibitor (trametinib).
Treatment Options for Childhood Chronic Myelogenous Leukemia
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment for childhood chronic myelogenous leukemia (CML) may include the following:
- Targeted therapy with a tyrosine kinase inhibitor (imatinib, dasatinib, or nilotinib).
Treatment of refractory or recurrent childhood CML may include the following:
- Targeted therapy with a tyrosine kinase inhibitor (dasatinib or nilotinib).
- Stem cell transplant using blood stem cells from a donor.
Treatment Options for Childhood Myelodysplastic Syndromes
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of childhood myelodysplastic syndromes (MDS) may include the following:
- Stem cell transplant using blood stem cells from a donor.
- Supportive care, including transfusion therapy and antibiotics.
- Lenalidomide therapy, for patients with certain gene changes.
- A clinical trial of targeted therapy.
If the MDS becomes acute myeloid leukemia (AML), treatment will be the same as treatment for newly diagnosed AML.
To Learn More About Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
For more information from the National Cancer Institute about childhood acute myeloid leukemia and other myeloid malignancies, see the following:
- Drugs Approved for Acute Myeloid Leukemia
- Drugs Approved for Myeloproliferative Neoplasms
- Blood-Forming Stem Cell Transplants
- Targeted Cancer Therapies
For more childhood cancer information and other general cancer resources, see the following:
- About Cancer
- Childhood Cancers
- CureSearch for Children’s Cancer
- Late Effects of Treatment for Childhood Cancer
- Adolescents and Young Adults with Cancer
- Children with Cancer: A Guide for Parents
- Cancer in Children and Adolescents
- Staging
- Coping with Cancer
- Questions to Ask Your Doctor about Cancer
- For Survivors and Caregivers
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute’s (NCI’s) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of childhood acute myeloid leukemia and other myeloid malignancies. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary (“Updated”) is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Pediatric Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become “standard.” Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI’s website. For more information, call the Cancer Information Service (CIS), NCI’s contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/leukemia/patient/child-aml-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389303]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.
Last Revised: 2019-04-12
If you want to know more about cancer and how it is treated, or if you wish to know about clinical trials for your type of cancer, you can call the NCI’s Cancer Information Service at 1-800-422-6237, toll free. A trained information specialist can talk with you and answer your questions.
Topic Contents
- General Information About Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
- Stages of Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
- Treatment Option Overview
- Treatment Options for Childhood Acute Myeloid Leukemia
- Treatment Options for Transient Abnormal Myelopoiesis or Children with Down Syndrome and AML
- Treatment Options for Childhood Acute Promyelocytic Leukemia
- Treatment Options for Juvenile Myelomonocytic Leukemia
- Treatment Options for Childhood Chronic Myelogenous Leukemia
- Treatment Options for Childhood Myelodysplastic Syndromes
- To Learn More About Childhood Acute Myeloid Leukemia and Other Myeloid Malignancies
- About This PDQ Summary
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.

