Childhood Ependymoma Treatment (PDQ®): Treatment – Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Ependymoma
Primary brain tumors, including ependymomas, are a diverse group of diseases that together constitute the most common solid tumor of childhood. Immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Brain tumors are classified according to histology, but tumor location and extent of spread are important factors that affect treatment and prognosis.
Ependymomas arise from ependymal cells that line the ventricles and passageways in the brain and the center of the spinal cord. Ependymal cells produce cerebrospinal fluid (CSF). These tumors are classified as supratentorial or infratentorial. In children, most ependymomas are infratentorial tumors that arise in or around the fourth ventricle. According to the 2016 revision to the World Health Organization (WHO) classification of tumors of the central nervous system, ependymal tumors are classified into the following five main subtypes:[1]
- Subependymoma (WHO Grade I).
- Myxopapillary ependymoma (WHO Grade I).
- Ependymoma (WHO Grade II).
- Ependymoma, RELA fusion–positive (WHO Grade II or Grade III).
- Anaplastic ependymoma (WHO Grade III).
The location of the tumor determines the clinical presentation. Treatment begins with surgery. The type of adjuvant therapy given, such as a second surgery, chemotherapy, or radiation therapy, depends on the following:
- Subtype of ependymoma.
- Whether the tumor was completely removed during the initial surgery.
- Whether the tumor has disseminated throughout the central nervous system.
- Child’s age.
The PDQ childhood brain tumor treatment summaries are organized primarily according to the WHO classification of nervous system tumors.[1] For a full description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, refer to the PDQ summary on Childhood Brain and Spinal Cord Tumors Treatment Overview.
Incidence
Childhood ependymoma comprises approximately 9% of all childhood brain tumors, representing about 200 cases per year in the United States.[2,3]
Anatomy
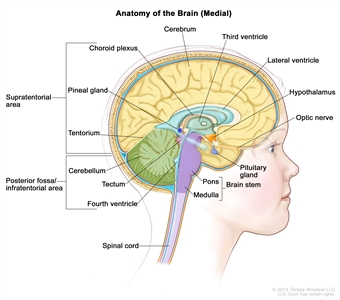
Figure 1. Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The tentorium separates the cerebrum from the cerebellum. The infratentorium (posterior fossa) is the region below the tentorium that contains the brain stem, cerebellum, and fourth ventricle. The supratentorium is the region above the tentorium and denotes the region that contains the cerebrum.
Molecular Features
Molecular characterization studies have identified several biological subtypes of ependymoma based on their distinctive DNA methylation and gene expression profiles and on their distinctive spectrum of genomic alterations (refer to Figure 2).[4,5,6]
- Infratentorial tumors.
- Posterior fossa A, CpG island methylator phenotype (CIMP)-positive ependymoma, termed EPN-PFA.
- Posterior fossa B, CIMP-negative ependymoma, termed EPN-PFB.
- Supratentorial tumors.
- C11orf95-RELA–positive ependymoma.
- C11orf95-RELA–negative and YAP1 fusion–positive ependymoma.
- Spinal tumors.
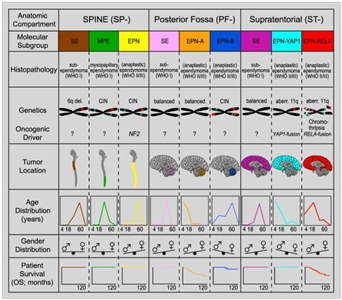
Figure 2. Graphical summary of key molecular and clinical characteristics of ependymal tumor subgroups. Schematic representation of key genetic and epigenetic findings in the nine molecular subgroups of ependymal tumors as identified by methylation profiling. CIN, Chromosomal instability. Reprinted from Cancer Cell, Volume 27, Kristian W. Pajtler, Hendrik Witt, Martin Sill, David T.W. Jones, Volker Hovestadt, Fabian Kratochwil, Khalida Wani, Ruth Tatevossian, Chandanamali Punchihewa, Pascal Johann, Juri Reimand, Hans-Jorg Warnatz, Marina Ryzhova, Steve Mack, Vijay Ramaswamy, David Capper, Leonille Schweizer, Laura Sieber, Andrea Wittmann, Zhiqin Huang, Peter van Sluis, Richard Volckmann, Jan Koster, Rogier Versteeg, Daniel Fults, Helen Toledano, Smadar Avigad, Lindsey M. Hoffman, Andrew M. Donson, Nicholas Foreman, Ekkehard Hewer, Karel Zitterbart, Mark Gilbert, Terri S. Armstrong, Nalin Gupta, Jeffrey C. Allen, Matthias A. Karajannis, David Zagzag, Martin Hasselblatt, Andreas E. Kulozik, Olaf Witt, V. Peter Collins, Katja von Hoff, Stefan Rutkowski, Torsten Pietsch, Gary Bader, Marie-Laure Yaspo, Andreas von Deimling, Peter Lichter, Michael D. Taylor, Richard Gilbertson, David W. Ellison, Kenneth Aldape, Andrey Korshunov, Marcel Kool, and Stefan M. Pfister, Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups, Pages 728–743, Copyright (2015), with permission from Elsevier.
Approximately two-thirds of childhood ependymomas arise in the posterior fossa, and two major genomically defined subtypes of posterior fossa tumors are recognized. Similarly, most pediatric supratentorial tumors can be categorized into one of two genomic subtypes. These subtypes and their associated clinical characteristics are described below.[4] Among these subtypes, the 2016 World Health Organization (WHO) classification has accepted ependymoma, RELA fusion–positive, as a distinct diagnostic entity.[7]
The most common posterior fossa ependymoma subtype is EPN-PFA and is characterized by the following:
- Presentation in young children (median age, 3 years).[4]
- Low rates of mutations that affect protein structure (approximately five per genome), with no recurring mutations.[5]
- A balanced chromosomal profile (refer to Figure 3) with few chromosomal gains or losses.[4,5]
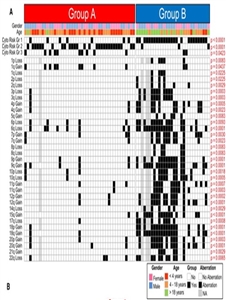
Figure 3. Identification of Subgroup-Specific Copy Number Alterations in the Posterior Fossa Ependymoma Genome. (A) Copy number profiling of 75 PF ependymomas using 10K array-CGH identifies disparate genetic landscapes between Group A and Group B tumors. Toronto and Heidelberg copy number datasets have been combined and summarized in a heatmap. The heatmap also displays the association of tumors to cytogenetic risk groups 1, 2, and 3 (Korshunov et al., 2010). Statistically significant chromosomal aberrations (black boxes) are also displayed between both subgroups, calculated by Fisher’s exact test. Witt H, Mack SC, Ryzhova M, et al.: Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20 (2): 143-57, 2011, doi:10.1016/j.ccr.2011.07.007. Copyright © 2011 Elsevier Inc. All rights reserved. - Gain of chromosome 1q, a known poor prognostic factor for ependymomas,[8] in approximately 25% of cases.[4,6]
- Presence of the CIMP (i.e., CIMP positive).[6]
- High rates of disease recurrence (33% progression-free survival [PFS] at 5 years) and low survival rates compared with other subtypes (68% at 5 years).[4]
The EPN-PFB subtype is less common than the EPN-PFA subtype in children and is characterized by the following:
- Presentation primarily in adolescents and young adults (median age, 30 years).[4]
- Low rates of mutations that affect protein structure (approximately five per genome), with no recurring mutations.[6]
- Numerous cytogenetic abnormalities (refer to Figure 3), primarily involving the gain/loss of whole chromosomes.[4,6]
- Absence of the CIMP (i.e., CIMP negative).[6]
- Favorable outcome in comparison to EPN-PFA, with 5-year PFS of 73% and overall survival (OS) of 100%.[4]
The largest subset of pediatric supratentorial (ST) ependymomas are characterized by gene fusions involving RELA,[9,10] a transcriptional factor important in NF-κB pathway activity. This subtype is termed ST-EPN-RELA and is characterized by the following:
- Represents approximately 70% of supratentorial ependymomas in children,[9,10] and presents at a median age of 8 years.[4]
- Presence of C11orf95-RELA fusions resulting from chromothripsis involving chromosome 11q13.1.[9]
- Evidence of NF-κB pathway activation at the protein and RNA level.[9]
- Low rates of mutations that affect protein structure and absence of recurring mutations outside of C11orf95-RELA fusions.[9]
- Presence of homozygous deletions of CDKN2A, a known poor prognostic factor for ependymomas,[8] in approximately 15% of cases.[4]
- Gain of chromosome 1q, a known poor prognostic factor for ependymomas, in approximately one-quarter of cases.[4]
- Unfavorable outcome in comparison to other ependymoma subtypes, with 5-year PFS of 29% and OS of 75%.[4]
- Supratentorial clear cell ependymomas with branching capillaries commonly show the C11orf95-RELA fusion,[11] and one series of 20 patients with a median age of 10.4 years showed a relatively favorable prognosis (5-year PFS of 68% and OS of 72%).[11]
A second, less common subset of supratentorial ependymomas, termed ST-EPN-YAP1, has fusions involving YAP1 and are characterized by the following:
- Median age at diagnosis of 1.4 years.[4]
- Presence of a gene fusion involving YAP1, with MAMLD1 being the most common fusion partner.[4,9]
- A relatively stable genome with few chromosomal changes other than the YAP1 fusion.[4]
- Relatively favorable prognosis (although based on small numbers), with a 5-year PFS of 66% and OS of 100%.[4]
Clinical implications of genomic alterations
The absence of recurring mutations in the EPN-PFA and EPN-PFB subtypes at diagnosis precludes using their genomic profiles to guide therapy. The RELA and YAP1 fusion genes present in supratentorial ependymomas are not directly targetable with agents in the clinic, but can provide leads for future research.
Clinical Features
The clinical presentation of ependymoma is dependent on tumor location.
- Infratentorial (posterior fossa) ependymoma: In children, approximately 65% to 75% of ependymomas arise in the posterior fossa.[12] Children with posterior fossa ependymoma may present with signs and symptoms of obstructive hydrocephalus due to obstruction at the level of the fourth ventricle. They may also present with ataxia, neck pain, or cranial nerve palsies.
- Supratentorial ependymoma: Supratentorial ependymoma may result in headache, seizures, or location-dependent focal neurologic deficits.
- Spinal cord ependymoma: Spinal cord ependymomas, which are often the myxopapillary variant, tend to cause back pain, lower extremity weakness, and/or bowel and bladder dysfunction.
Diagnostic Evaluation
Every patient suspected of having ependymoma is evaluated with diagnostic imaging of the whole brain and spinal cord. The most sensitive method available for evaluating spinal cord subarachnoid metastasis is spinal magnetic resonance imaging (MRI) performed with gadolinium. This is ideally done before surgery to avoid confusion with postoperative blood. If MRI is used, the entire spine is generally imaged in at least two planes with contiguous MRI slices performed after gadolinium enhancement.
If feasible, CSF cytological evaluation is conducted.[13]
Prognostic Factors
Unfavorable factors affecting outcome (except as noted) include the following:
- Molecular characteristics.
Posterior fossa ependymoma can be divided into the following two groups based on distinctive patterns of gene expression.[4,5,14,15]
- EPN-PFA occurs primarily in young children and is characterized by a largely balanced genomic profile with an increased occurrence of chromosome 1q gain [8,16,17,18] and expression of genes and proteins previously shown to be associated with poor prognosis, such as tenascin C and epidermal growth factor receptor.[16,19,20]
- In contrast, EPN-PFB occurs primarily in older children and adults and is characterized by a more favorable prognosis and by numerous cytogenetic abnormalities involving whole chromosomes or chromosomal arms.[14]
Chromosome 1q25 gain, which is present in approximately 20% of ependymoma cases,[8,17] is reported to be prognostic for inferior outcome for both posterior fossa ependymoma and supratentorial ependymoma.[20] Other factors that have been reported to be associated with poor prognosis for pediatric ependymoma include expression of the enzymatic subunit of telomerase (hTERT) [21,22,23] and expression of the neural stem cell marker Nestin.[24][Level of evidence: 3iiiA]
- Tumor location. Cranial variants of ependymoma have a less favorable outcome than primary spinal cord ependymomas.[25,26] Location within the spinal cord may also affect outcome, with tumors in the lower portion of the spinal cord having a worse prognosis.[27][Level of evidence: 3iiiA]
- Younger age at diagnosis.[28][Level of evidence: 3iiiDii]
- Anaplastic histology.[29][Level of evidence: 2A]; [28,30,31]; [32][Level of evidence: 3iA]; [33][Level of evidence: 3iiiDi]
- Subtotal resection.[28]; [29][Level of evidence: 2A]
- Lower doses of radiation.[34]
- Immunohistochemical testing has identified increased expression of markers of proliferation (e.g., Ki-67 and MIB-1) [35,36] and increased expression of EZH2, a polycomb complex protein involved in epigenetic regulation of gene expression, as prognostic factors for greater risk of treatment failure.[37]
Follow-up After Treatment
Surveillance neuroimaging, coupled with clinical assessments, are generally recommended after treatment for ependymoma. The frequency and duration have been arbitrarily determined and the utility is uncertain.[38] Most practitioners obtain MRI imaging of the brain and/or spinal cord every 3 months for the first 1 to 2 years after treatment. After 2 years, imaging every 6 months for the next 3 years is often undertaken.
References:
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. Lyon, France: IARC Press, 2016.
- Gurney JG, Smith MA, Bunin GR: CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, Chapter 3, pp 51-63. Also available online. Last accessed October 12, 2018.
- Central Brain Tumor Registry of the United States: Statistical Report: Primary Brain Tumors in the United States, 1997-2001. Hinsdale, Ill: Central Brain Tumor Registry of the United States, 2004. Also available online. Last accessed October 12, 2018.
- Pajtler KW, Witt H, Sill M, et al.: Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 27 (5): 728-43, 2015.
- Witt H, Mack SC, Ryzhova M, et al.: Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20 (2): 143-57, 2011.
- Mack SC, Witt H, Piro RM, et al.: Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 506 (7489): 445-50, 2014.
- Louis DN, Perry A, Reifenberger G, et al.: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6): 803-20, 2016.
- Korshunov A, Witt H, Hielscher T, et al.: Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol 28 (19): 3182-90, 2010.
- Parker M, Mohankumar KM, Punchihewa C, et al.: C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506 (7489): 451-5, 2014.
- Pietsch T, Wohlers I, Goschzik T, et al.: Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol 127 (4): 609-11, 2014.
- Figarella-Branger D, Lechapt-Zalcman E, Tabouret E, et al.: Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro Oncol 18 (7): 919-27, 2016.
- Andreiuolo F, Puget S, Peyre M, et al.: Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol 12 (11): 1126-34, 2010.
- Moreno L, Pollack IF, Duffner PK, et al.: Utility of cerebrospinal fluid cytology in newly diagnosed childhood ependymoma. J Pediatr Hematol Oncol 32 (6): 515-8, 2010.
- Wani K, Armstrong TS, Vera-Bolanos E, et al.: A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123 (5): 727-38, 2012.
- Ramaswamy V, Hielscher T, Mack SC, et al.: Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. J Clin Oncol 34 (21): 2468-77, 2016.
- Mendrzyk F, Korshunov A, Benner A, et al.: Identification of gains on 1q and epidermal growth factor receptor overexpression as independent prognostic markers in intracranial ependymoma. Clin Cancer Res 12 (7 Pt 1): 2070-9, 2006.
- Kilday JP, Mitra B, Domerg C, et al.: Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children’s Cancer Leukaemia Group (CCLG), Societe Francaise d’Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP). Clin Cancer Res 18 (7): 2001-11, 2012.
- Godfraind C, Kaczmarska JM, Kocak M, et al.: Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol 124 (2): 247-57, 2012.
- Korshunov A, Golanov A, Timirgaz V: Immunohistochemical markers for intracranial ependymoma recurrence. An analysis of 88 cases. J Neurol Sci 177 (1): 72-82, 2000.
- Andreiuolo F, Le Teuff G, Bayar MA, et al.: Integrating Tenascin-C protein expression and 1q25 copy number status in pediatric intracranial ependymoma prognostication: A new model for risk stratification. PLoS One 12 (6): e0178351, 2017.
- Tabori U, Ma J, Carter M, et al.: Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J Clin Oncol 24 (10): 1522-8, 2006.
- Tabori U, Wong V, Ma J, et al.: Telomere maintenance and dysfunction predict recurrence in paediatric ependymoma. Br J Cancer 99 (7): 1129-35, 2008.
- Modena P, Buttarelli FR, Miceli R, et al.: Predictors of outcome in an AIEOP series of childhood ependymomas: a multifactorial analysis. Neuro Oncol 14 (11): 1346-56, 2012.
- Milde T, Hielscher T, Witt H, et al.: Nestin expression identifies ependymoma patients with poor outcome. Brain Pathol 22 (6): 848-60, 2012.
- McGuire CS, Sainani KL, Fisher PG: Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer 52 (1): 65-9, 2009.
- Benesch M, Frappaz D, Massimino M: Spinal cord ependymomas in children and adolescents. Childs Nerv Syst 28 (12): 2017-28, 2012.
- Oh MC, Sayegh ET, Safaee M, et al.: Prognosis by tumor location for pediatric spinal cord ependymomas. J Neurosurg Pediatr 11 (3): 282-8, 2013.
- Tamburrini G, D’Ercole M, Pettorini BL, et al.: Survival following treatment for intracranial ependymoma: a review. Childs Nerv Syst 25 (10): 1303-12, 2009.
- Massimino M, Miceli R, Giangaspero F, et al.: Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol 18 (10): 1451-60, 2016.
- Merchant TE, Jenkins JJ, Burger PC, et al.: Influence of tumor grade on time to progression after irradiation for localized ependymoma in children. Int J Radiat Oncol Biol Phys 53 (1): 52-7, 2002.
- Korshunov A, Golanov A, Sycheva R, et al.: The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: an analysis of 258 patients. Cancer 100 (6): 1230-7, 2004.
- Amirian ES, Armstrong TS, Aldape KD, et al.: Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology 39 (2): 116-24, 2012.
- Tihan T, Zhou T, Holmes E, et al.: The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol 21 (2): 165-77, 2008.
- Vaidya K, Smee R, Williams JR: Prognostic factors and treatment options for paediatric ependymomas. J Clin Neurosci 19 (9): 1228-35, 2012.
- Wolfsberger S, Fischer I, Höftberger R, et al.: Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol 28 (7): 914-20, 2004.
- Kurt E, Zheng PP, Hop WC, et al.: Identification of relevant prognostic histopathologic features in 69 intracranial ependymomas, excluding myxopapillary ependymomas and subependymomas. Cancer 106 (2): 388-95, 2006.
- Li AM, Dunham C, Tabori U, et al.: EZH2 expression is a prognostic factor in childhood intracranial ependymoma: a Canadian Pediatric Brain Tumor Consortium study. Cancer 121 (9): 1499-507, 2015.
- Good CD, Wade AM, Hayward RD, et al.: Surveillance neuroimaging in childhood intracranial ependymoma: how effective, how often, and for how long? J Neurosurg 94 (1): 27-32, 2001.
Histopathologic Classification of Childhood Ependymal Tumors
For the first time, the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) incorporates the addition of genotypic findings in the classification of select CNS tumors. This integrated classification is intended to define more homogeneous entities that will improve the accuracy of diagnoses, refine prognoses, and more reliably reach conclusions regarding treatment strategies.
Ependymal tumors are now classified into the following five main subtypes:[1]
- Subependymoma (WHO Grade I): A subependymoma is a slow-growing neoplasm, typically attached to the ventricle wall and is composed of glial tumor cell clusters embedded in a fibrillary matrix.
The true incidence of subependymomas (WHO Grade I) is difficult to determine. These tumors are frequently asymptomatic and may be found incidentally at autopsy. Subependymomas probably comprise less than 5% of all ependymal tumors.
- Myxopapillary ependymoma (WHO Grade I): A myxopapillary ependymoma arises almost exclusively in the location of the conus medullaris, cauda equina, and filum terminale of the spinal cord and is characterized histologically by tumor cells arranged in a papillary manner around vascularized myxoid stromal cores.
- Ependymoma (WHO Grade II): The ependymoma, which is considered a Grade II neoplasm originating from the walls of the ventricles or from the spinal canal, is composed of neoplastic ependymal cells. In the 2016 WHO revision, the term cellular ependymoma was eliminated as a subtype because it was felt to be synonymous with standard ependymoma. Three additional subtypes of ependymoma WHO Grade II tumors include the following:
- Papillary ependymoma—forms linear, epithelial-like surfaces along cerebrospinal fluid exposures.
- Clear cell ependymoma—displays an oligodendroglial-like appearance with perinuclear halos; this variant is preferentially located in the supratentorial compartment of the brain.
- Tanycytic ependymoma—the rarest form of Grade II ependymoma; this subtype is most commonly found in the spinal cord; tumor cells are arranged in fascicles of variable width and cell density and are poorly intertwined.
- Ependymoma, RELA fusion–positive (WHO Grade II or Grade III): This integrated diagnosis is seen in most supratentorial ependymal tumors in children. Phenotypically, it is similar to ependymoma (WHO Grade II) or anaplastic ependymoma (WHO Grade III). These tumors are characterized by a C11orf95-RELA fusion, and L1CAM immunohistochemistry may serve as a surrogate for this subtype.[2]
- Anaplastic ependymoma (WHO Grade III): Also known as malignant ependymoma. An anaplastic ependymoma is considered a malignant glioma of ependymal differentiation and, compared with the Grade II ependymomas, shows increased cellularity and increased mitotic activity, often associated with microvascular proliferation and necrosis.
Subependymomas and myxopapillary ependymomas are usually considered to be clinically and pathologically distinct from the Grade II and Grade III ependymomas.
Although supratentorial and infratentorial ependymomas are believed to arise from radial glia cells, they have different genomic, gene expression, and immunohistochemical signatures.[3,4,5] Supratentorial tumors are more often characterized by neuronal differentiation.[4]
Ependymoblastoma is no longer recognized in the WHO classification and is now classified as an embryonal tumor with multilayered rosettes.
The pathologic classification of pediatric brain tumors is a specialized area that is evolving; review of the diagnostic tissue by a neuropathologist who has particular expertise in this area is strongly recommended.
References:
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. Lyon, France: IARC Press, 2016.
- Parker M, Mohankumar KM, Punchihewa C, et al.: C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506 (7489): 451-5, 2014.
- Taylor MD, Poppleton H, Fuller C, et al.: Radial glia cells are candidate stem cells of ependymoma. Cancer Cell 8 (4): 323-35, 2005.
- Andreiuolo F, Puget S, Peyre M, et al.: Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol 12 (11): 1126-34, 2010.
- Grill J, Bergthold G, Ferreira C: Pediatric ependymomas: will molecular biology change patient management? Curr Opin Oncol 23 (6): 638-42, 2011.
Stage Information for Childhood Ependymoma
Although there is no formal staging system, ependymomas can be divided into supratentorial, infratentorial, and spinal tumors. Approximately 30% of childhood ependymomas arise in supratentorial regions of the brain and 70% arise in the posterior fossa.[1] They usually originate in the ependymal linings of ventricles or central canal or ventriculus terminalis of the spinal cord and have access to the cerebrospinal fluid. Therefore, these tumors may spread throughout the neuraxis, although dissemination is noted in less than 10% of patients with Grade II and Grade III ependymomas. Myxopapillary ependymomas are more likely to disseminate to the nervous system early in the course of illness.
References:
- Villano JL, Parker CK, Dolecek TA: Descriptive epidemiology of ependymal tumours in the United States. Br J Cancer 108 (11): 2367-71, 2013.
Treatment Option Overview for Childhood Ependymoma
Many of the improvements in survival in childhood cancer have been made as a result of clinical trials that have attempted to improve on the best available, accepted therapy. Clinical trials in pediatrics are designed to compare new therapy with therapy that is currently accepted as standard. This comparison may be done in a randomized study of two treatment arms or by evaluating a single new treatment and comparing the results with those previously obtained with existing therapy.
Because of the relative rarity of cancer in children, all patients with aggressive brain tumors should be considered for entry into a clinical trial. To determine and implement optimum treatment, treatment planning by a multidisciplinary team of cancer specialists who have experience treating childhood brain tumors is required. Radiation therapy of pediatric brain tumors is technically demanding and should be performed in centers that have experience in that area to ensure optimal results.
Table 1 describes the standard treatment options for newly diagnosed and recurrent childhood ependymoma.
| Treatment Group | Standard Treatment Options | |
|---|---|---|
| Newly diagnosed childhood subependymoma | Surgery | |
| Observation (in rare cases) | ||
| Newly diagnosed childhood myxopapillary ependymoma | Surgery with or without radiation therapy | |
| Newly diagnosed childhood ependymoma (WHO Grade II), anaplastic ependymoma (WHO Grade III), orRELAfusion–positive ependymoma: | Surgery | |
| Adjuvant therapy: | ||
| No residual disease, no disseminated disease | — Radiation therapy | |
| Residual disease, no disseminated disease | — Second-look surgery | |
| — Radiation therapy | ||
| — Preirradiation chemotherapy | ||
| Central nervous system disseminated disease | — Radiation therapy | |
| Children younger than 3 years | — Chemotherapy | |
| — Radiation therapy | ||
| Recurrent childhood ependymoma | Surgery | |
| Radiation therapy and/or chemotherapy | ||
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
References:
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
Treatment of Newly Diagnosed Childhood Subependymoma
Subependymomas are exceedingly rare in children and approaches to treatment have been inferred from the experience in the adult population.
The standard treatment options for newly diagnosed subependymoma (WHO Grade I) include the following:
- Surgery.
- Observation (in rare cases).
In cases requiring therapy, complete surgical removal is often curative. Some subependymomas are considered incidental findings and observed without intervention.
Occasionally, subependymomas cause ventricular obstruction and, in these cases, ventriculoperitoneal shunt placement is indicated. Spontaneous intratumoral hemorrhage has also been observed.[1]
References:
- Jain A, Amin AG, Jain P, et al.: Subependymoma: clinical features and surgical outcomes. Neurol Res 34 (7): 677-84, 2012.
Treatment of Newly Diagnosed Childhood Myxopapillary Ependymoma
Myxopapillary ependymomas, considered to be a histologic subtype of ependymoma, have a relatively high incidence of central nervous system tumor dissemination at diagnosis and at follow-up. Imaging of the complete craniospinal axis at the time of diagnosis and during follow-up is indicated.[1,2]
Standard treatment options for newly diagnosed myxopapillary ependymoma (WHO Grade I) include the following:
- Surgery with or without adjuvant radiation therapy.
Historically, the management of myxopapillary ependymoma (WHO Grade I) consisted of an attempt at en bloc resection of the tumor with no further treatment in the case of a gross-total resection.[3]; [4][Level of evidence: 3iiiDi] However, based on the finding that dissemination of these tumors to other parts of the neuraxis can occur, particularly when complete resection is not obtained, and evidence that focal radiation therapy may improve progression-free survival, many practitioners now favor the use of radiation therapy after surgical resection of the primary mass.[1,3]; [5][Level of evidence: 3iiiDi]; [6,7][Level of evidence: 3iiiDiii]
References:
- Fassett DR, Pingree J, Kestle JR: The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients. Report of five cases and review of the literature. J Neurosurg 102 (1 Suppl): 59-64, 2005.
- Bagley CA, Kothbauer KF, Wilson S, et al.: Resection of myxopapillary ependymomas in children. J Neurosurg 106 (4 Suppl): 261-7, 2007.
- Akyurek S, Chang EL, Yu TK, et al.: Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol 80 (2): 177-83, 2006.
- Bagley CA, Wilson S, Kothbauer KF, et al.: Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg Rev 32 (3): 321-34; discussion 334, 2009.
- Pica A, Miller R, Villà S, et al.: The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the rare cancer network. Int J Radiat Oncol Biol Phys 74 (4): 1114-20, 2009.
- Agbahiwe HC, Wharam M, Batra S, et al.: Management of pediatric myxopapillary ependymoma: the role of adjuvant radiation. Int J Radiat Oncol Biol Phys 85 (2): 421-7, 2013.
- Jeibmann A, Egensperger R, Kuchelmeister K, et al.: Extent of surgical resection but not myxopapillary versus classical histopathological subtype affects prognosis in lumbo-sacral ependymomas. Histopathology 54 (2): 260-2, 2009.
Treatment of Newly Diagnosed Childhood Ependymoma, Anaplastic Ependymoma, or RELA Fusion–Positive Ependymoma
Standard treatment options for newly diagnosed ependymoma (WHO Grade II), anaplastic ependymoma (WHO Grade III), or RELA fusion–positive ependymoma include the following:
- Surgery.
- Adjuvant therapy.
- Treatment options for no residual disease, no disseminated disease.
- Treatment options for residual disease, no disseminated disease.
- Treatment options for central nervous system (CNS) disseminated disease.
- Treatment options for children younger than 3 years.
Typically, all patients undergo surgery to remove the tumor. Whether additional treatment is given depends on the extent of tumor resection and whether there is disseminated disease.
Surgery
Surgery is performed in an attempt at maximal tumor reduction. Evidence suggests that more extensive surgical resection is related to an improved rate of survival.[1,2,3,4,5]; [6,7][Level of evidence: 3iDii] Magnetic resonance imaging (MRI) is performed postoperatively to confirm the extent of resection. If not performed preoperatively, MRI of the entire neuraxis to evaluate disease dissemination and cerebrospinal fluid cytopathology is performed.
Patients with residual tumor or disseminated disease should be considered at high risk of relapse and may be treated on protocols specifically designed for them. Those with no evidence of residual tumor still have an approximate 20% to 40% relapse risk in spite of postoperative radiation therapy.
Anecdotal experience suggests that surgery alone for completely resected supratentorial nonanaplastic tumors and intradural spinal cord ependymomas may, in select cases, be an appropriate approach to treatment.[8,9][Level of evidence: 3iiiDi]; [10,11,12][Level of evidence: 3iiiDiii]
Retrospective analysis of the outcome for patients with posterior fossa ependymoma (EPN-PFB) suggests that these patients might be sufficiently treated with gross-total resection alone,[7] but this approach has not been tested in a prospective, randomized clinical trial.
Adjuvant Therapy
Treatment options for no residual disease, no disseminated disease
Radiation therapy
The traditional postsurgical treatment for these patients has been radiation therapy consisting of 54 Gy to 59.4 Gy to the tumor bed for children aged 3 years and older.[5,13] It is not necessary to treat the entire CNS (whole brain and spine) because these tumors usually recur initially at the local site.[14]; [15][Level of evidence: 3iiiA] When possible, patients should be treated in a center experienced with the delivery of highly conformal radiation therapy (including intensity-modulated radiation therapy or charged-particle radiation therapy) to pediatric patients with brain tumors.
Evidence (radiation therapy):
- In one study, 74 patients aged 1 to 21 years were treated with conformal radiation therapy after surgery.[16]
- The 3-year progression-free survival (PFS) rate was 77.6% ± 5.8%.
- In a second series, 107 of 153 patients received conformal radiation therapy immediately after up-front resection.[5][Level of evidence: 3iA]
- The 7-year event-free survival was 76.9% ± 13.5%.
- Focal radiation therapy has been used in certain cases.[17] In a small series of children with localized ependymoma, surgery alone was compared with adjuvant radiation therapy.[18]
- Adjuvant radiation therapy appeared to improve PFS, even after adjusting for the extent of resection. In fact, a PFS benefit was observed for patients who received adjuvant radiation therapy after gross-total resection, compared with those who did not receive radiation therapy.
- Additional research will be necessary to confirm these findings.
- Proton-beam radiation therapy (a type of charged-particle radiation therapy) provides a possible advantage for targeting the tumor (supratentorial or infratentorial) while avoiding critical normal brain and neuroendocrine tissues. The 3-year PFS rate for children treated with proton-beam radiation therapy was 76% in two series and appears to be similar to the PFS rate for children treated with intensity-modulated photon-beam radiation therapy.[19,20,21] In the two series that used proton-beam radiation therapy, the 3-year local control rates were 83% to 85%, with overall survival rates of 90% to 95%.[19,20]
Concerns of brain stem toxicity in very young children (aged <3 years) after proton therapy to the posterior fossa have prompted the use of more conservative doses in these children at some centers.[19,21,22]
Chemotherapy
There is no evidence to date that adjuvant chemotherapy, including the use of myeloablative chemotherapy,[23] improves the outcome for patients with totally resected, nondisseminated ependymoma. For this reason, current treatment approaches do not include chemotherapy as a standard component of primary therapy for children with newly diagnosed ependymomas that are completely resected.
A randomized trial evaluating the efficacy of postradiation chemotherapy in children who have had a gross-total resection is under way.
Treatment options for residual disease, no disseminated disease
Second-look surgery
Second-look surgery should be considered because patients who have complete resections have better disease control.[24] In some cases, further surgery can be undertaken after the initial attempted resection if the pediatric neurosurgeon believes that a gross-total resection could be obtained by an alternate surgical approach to the tumor. In other cases, further up-front surgery is not anticipated to result in a gross-total resection, therefore, adjuvant therapy is initiated with future consideration of second-look surgery.
Radiation therapy
The rationale for radiation therapy as described in the Treatment options for no residual disease, no disseminated disease subsection above also pertains to the treatment of children with residual, nondisseminated ependymoma. In patients with a subtotal resection, treatment with radiation therapy results in 3-year to 5-year PFS in 30% to 50% of patients,[16] although the outcome for patients with residual tumor within the spinal canal may be better.[25]
Preirradiation chemotherapy
One study demonstrated a benefit of preirradiation chemotherapy in children with near-total resection (>90% resection), with outcomes similar to children achieving a gross-total resection followed by radiation therapy.[26] The Children’s Oncology Group (COG) has completed a study of preirradiation chemotherapy in children with residual disease after up-front surgery to determine whether children treated with chemotherapy can achieve a complete response with chemotherapy or second-look surgery. Results are pending.
There is no evidence that high-dose chemotherapy with stem cell rescue is of any benefit.[27]; [28][Level of evidence: 2A]
Treatment options for CNS disseminated disease
Radiation therapy
Regardless of the degree of surgical resection, these patients generally receive radiation therapy to the whole brain and spine, along with boosts to local disease and bulk areas of disseminated disease. The traditional local postsurgical radiation doses in these patients have been 54 Gy to 55.8 Gy. Doses of approximately 36 Gy to the entire neuraxis (i.e., the whole brain and spine) are also administered but may be modulated depending on the age of the patient.[29] Boosts between 41.4 Gy and 50.4 Gy to bulk areas of spinal disease are administered, with doses depending on the age of the patient and the location of the tumor. However, there are no contemporary studies published to support this approach.
Chemotherapy
The role of chemotherapy in the management of children with disseminated ependymoma is unproven.[30]
Treatment options for children younger than 3 years
Chemotherapy
Some, but not all, chemotherapy regimens induce objective responses in children younger than 3 years with newly diagnosed ependymoma.[31,32,33,34] Up to 40% of infants and young children with totally resected disease may achieve long-term survival with chemotherapy alone.[35][Level of evidence: 2Di]
Radiation therapy
Historically, postoperative radiation therapy was omitted for children younger than 3 years with ependymoma. The previous two COG studies have lowered the age limit for postoperative radiation therapy to age 1 year in an effort to improve outcomes for these younger children. The first of these two studies (ACNS0121 [NCT00027846]) is awaiting publication to provide evidence of the utility of this approach.
Evidence (radiation therapy):
- Retrospective reviews based on Surveillance, Epidemiology, and End Results data of children younger than 3 years at diagnosis were accrued over a 50-year period.[36]
- Results showed that patients who received local radiation therapy had better 10-year survival rates, even after adjusting for extent of resection and tumor grade (grade 2 vs. grade 3).
- Conformal radiation therapy is an alternative approach for minimizing radiation-induced neurologic damage in young children with ependymoma. The need and timing of radiation therapy for children who have successfully completed chemotherapy and have no residual disease is still to be determined.
- The initial experience with this approach suggested that children younger than 3 years with ependymoma have neurologic deficits at diagnosis that improve with time after conformal radiation treatment.[16]
- Another study suggested that there was a trend for intellectual deterioration over time even in older children treated with localized radiation therapy.[37][Level of evidence: 3iiiC]
Conformal radiation approaches, such as 3-dimensional conformal radiation therapy, that minimize damage to normal brain tissue and charged-particle radiation therapy, such as proton-beam therapy, are under evaluation for infants and children with ependymoma.[16,38] When analyzing neurologic outcome after treatment of young children with ependymoma, it is important to consider that not all long-term deficits can be ascribed to radiation therapy because deficits may be present in young children before therapy begins.[16] For example, the presence of hydrocephalus at diagnosis is associated with lower intelligence quotient as measured after surgical resection and before administration of radiation therapy.[39]
The recently closed COG protocol (ACNS0121 [NCT00027846]) for children with ependymoma includes children aged 1 year and older. The trial is a prospective evaluation of postoperative radiation therapy. Results are forthcoming.
Treatment Options Under Clinical Evaluation for Newly Diagnosed Childhood Ependymoma or Anaplastic Ependymoma
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- COG-ACNS0831 (NCT01096368) (Maintenance Chemotherapy or Observation Following Induction Chemotherapy and Radiation Therapy in Treating Younger Patients With Newly Diagnosed Ependymoma): The purpose of this phase III trial is as follows:
No Residual Disease; No Disseminated Disease
- The trial will determine whether adding chemotherapy after radiation therapy results in improved survival over radiation therapy alone.
- The trial will determine whether children with supratentorial nonanaplastic ependymoma who receive a complete resection or who achieve a complete remission after being treated with chemotherapy can be successfully treated without radiation therapy.
Residual Disease; No Disseminated Disease
- The trial will determine whether adding chemotherapy before and after radiation therapy results in improved survival compared with previous studies of children who did not receive additional chemotherapy after radiation treatment.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- van Veelen-Vincent ML, Pierre-Kahn A, Kalifa C, et al.: Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J Neurosurg 97 (4): 827-35, 2002.
- Abdel-Wahab M, Etuk B, Palermo J, et al.: Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys 64 (4): 1060-71, 2006.
- Kothbauer KF: Neurosurgical management of intramedullary spinal cord tumors in children. Pediatr Neurosurg 43 (3): 222-35, 2007.
- Zacharoulis S, Ji L, Pollack IF, et al.: Metastatic ependymoma: a multi-institutional retrospective analysis of prognostic factors. Pediatr Blood Cancer 50 (2): 231-5, 2008.
- Merchant TE, Li C, Xiong X, et al.: Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 10 (3): 258-66, 2009.
- Cage TA, Clark AJ, Aranda D, et al.: A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. J Neurosurg Pediatr 11 (6): 673-81, 2013.
- Ramaswamy V, Hielscher T, Mack SC, et al.: Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. J Clin Oncol 34 (21): 2468-77, 2016.
- Volpp PB, Han K, Kagan AR, et al.: Outcomes in treatment for intradural spinal cord ependymomas. Int J Radiat Oncol Biol Phys 69 (4): 1199-204, 2007.
- Hukin J, Epstein F, Lefton D, et al.: Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg 29 (1): 40-5, 1998.
- Little AS, Sheean T, Manoharan R, et al.: The management of completely resected childhood intracranial ependymoma: the argument for observation only. Childs Nerv Syst 25 (3): 281-4, 2009.
- Venkatramani R, Dhall G, Patel M, et al.: Supratentorial ependymoma in children: to observe or to treat following gross total resection? Pediatr Blood Cancer 58 (3): 380-3, 2012.
- Ghia AJ, Mahajan A, Allen PK, et al.: Supratentorial gross-totally resected non-anaplastic ependymoma: population based patterns of care and outcomes analysis. J Neurooncol 115 (3): 513-20, 2013.
- Koshy M, Rich S, Merchant TE, et al.: Post-operative radiation improves survival in children younger than 3 years with intracranial ependymoma. J Neurooncol 105 (3): 583-90, 2011.
- Combs SE, Kelter V, Welzel T, et al.: Influence of radiotherapy treatment concept on the outcome of patients with localized ependymomas. Int J Radiat Oncol Biol Phys 71 (4): 972-8, 2008.
- Schroeder TM, Chintagumpala M, Okcu MF, et al.: Intensity-modulated radiation therapy in childhood ependymoma. Int J Radiat Oncol Biol Phys 71 (4): 987-93, 2008.
- Merchant TE, Mulhern RK, Krasin MJ, et al.: Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 22 (15): 3156-62, 2004.
- Landau E, Boop FA, Conklin HM, et al.: Supratentorial ependymoma: disease control, complications, and functional outcomes after irradiation. Int J Radiat Oncol Biol Phys 85 (4): e193-9, 2013.
- Pejavar S, Polley MY, Rosenberg-Wohl S, et al.: Pediatric intracranial ependymoma: the roles of surgery, radiation and chemotherapy. J Neurooncol 106 (2): 367-75, 2012.
- Indelicato DJ, Bradley JA, Rotondo RL, et al.: Outcomes following proton therapy for pediatric ependymoma. Acta Oncol 57 (5): 644-648, 2018.
- Macdonald SM, Sethi R, Lavally B, et al.: Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol 15 (11): 1552-9, 2013.
- Sato M, Gunther JR, Mahajan A, et al.: Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 123 (13): 2570-2578, 2017.
- Indelicato DJ, Flampouri S, Rotondo RL, et al.: Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 53 (10): 1298-304, 2014.
- Zacharoulis S, Levy A, Chi SN, et al.: Outcome for young children newly diagnosed with ependymoma, treated with intensive induction chemotherapy followed by myeloablative chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer 49 (1): 34-40, 2007.
- Massimino M, Solero CL, Garrè ML, et al.: Second-look surgery for ependymoma: the Italian experience. J Neurosurg Pediatr 8 (3): 246-50, 2011.
- Wahab SH, Simpson JR, Michalski JM, et al.: Long term outcome with post-operative radiation therapy for spinal canal ependymoma. J Neurooncol 83 (1): 85-9, 2007.
- Garvin JH Jr, Selch MT, Holmes E, et al.: Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children’s Cancer Group protocol 9942: a report from the Children’s Oncology Group. Pediatr Blood Cancer 59 (7): 1183-9, 2012.
- Grill J, Kalifa C, Doz F, et al.: A high-dose busulfan-thiotepa combination followed by autologous bone marrow transplantation in childhood recurrent ependymoma. A phase-II study. Pediatr Neurosurg 25 (1): 7-12, 1996.
- Venkatramani R, Ji L, Lasky J, et al.: Outcome of infants and young children with newly diagnosed ependymoma treated on the “Head Start” III prospective clinical trial. J Neurooncol 113 (2): 285-91, 2013.
- Merchant TE, Boop FA, Kun LE, et al.: A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 71 (1): 87-97, 2008.
- Bouffet E, Capra M, Bartels U: Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst 25 (10): 1293-301, 2009.
- Duffner PK, Horowitz ME, Krischer JP, et al.: The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro-oncol 1 (2): 152-61, 1999.
- Duffner PK, Horowitz ME, Krischer JP, et al.: Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328 (24): 1725-31, 1993.
- Geyer JR, Sposto R, Jennings M, et al.: Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol 23 (30): 7621-31, 2005.
- Grill J, Le Deley MC, Gambarelli D, et al.: Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol 19 (5): 1288-96, 2001.
- Grundy RG, Wilne SA, Weston CL, et al.: Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol 8 (8): 696-705, 2007.
- Snider CA, Yang K, Mack SC, et al.: Impact of radiation therapy and extent of resection for ependymoma in young children: A population-based study. Pediatr Blood Cancer 65 (3): , 2018.
- von Hoff K, Kieffer V, Habrand JL, et al.: Impairment of intellectual functions after surgery and posterior fossa irradiation in children with ependymoma is related to age and neurologic complications. BMC Cancer 8: 15, 2008.
- MacDonald SM, Safai S, Trofimov A, et al.: Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys 71 (4): 979-86, 2008.
- Merchant TE, Lee H, Zhu J, et al.: The effects of hydrocephalus on intelligence quotient in children with localized infratentorial ependymoma before and after focal radiation therapy. J Neurosurg 101 (2 Suppl): 159-68, 2004.
Treatment of Recurrent Childhood Ependymoma
Recurrence is not uncommon for all grades of ependymoma and may develop many years after initial treatment.[1] Late recurrence beyond 10 to 15 years has been reported.[2] Disease generally recurs at the primary tumor site, although concomitant neuraxis dissemination may also be seen. Systemic relapse is extremely rare. At time of relapse, a complete evaluation for extent of recurrence is indicated for all patients.
Treatment options for recurrent childhood ependymoma include the following:
- Surgery.
- Radiation therapy and/or chemotherapy.
Surgery
The need for further surgical intervention is individualized based on the following:
- Extent of the tumor.
- Length of time between initial treatment and the reappearance of the recurrent lesion.
In some cases, surgically accessible lesions may be treated alternatively by radiation therapy.
Radiation Therapy and/or Chemotherapy
Patients with recurrent ependymomas should be considered for treatment with the following modalities:[3][Level of evidence: 3iiiB]
- Focal retreatment with various radiation modalities, including stereotactic radiosurgery,[4,5][Level of evidence: 3iiiA]; [6,7][Level of evidence: 3iiiDi] intensity-modulated photon therapy, and proton therapy.[8][Level of evidence: 3iiiB]
- Active anticancer agents, including cyclophosphamide, cisplatin, carboplatin, lomustine, and etoposide.
Regardless of treatment strategy, the prognosis for patients with recurrence is poor.[1] Entry into studies of novel therapeutic approaches should be considered.
Treatment Options Under Clinical Evaluation for Recurrent Childhood Ependymoma
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children’s Oncology Group (COG), the Pediatric Brain Tumor Consortium, or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Zacharoulis S, Ashley S, Moreno L, et al.: Treatment and outcome of children with relapsed ependymoma: a multi-institutional retrospective analysis. Childs Nerv Syst 26 (7): 905-11, 2010.
- Wu J, Armstrong TS, Gilbert MR: Biology and management of ependymomas. Neuro Oncol 18 (7): 902-13, 2016.
- Messahel B, Ashley S, Saran F, et al.: Relapsed intracranial ependymoma in children in the UK: patterns of relapse, survival and therapeutic outcome. Eur J Cancer 45 (10): 1815-23, 2009.
- Kano H, Yang HC, Kondziolka D, et al.: Stereotactic radiosurgery for pediatric recurrent intracranial ependymomas. J Neurosurg Pediatr 6 (5): 417-23, 2010.
- Bouffet E, Hawkins CE, Ballourah W, et al.: Survival benefit for pediatric patients with recurrent ependymoma treated with reirradiation. Int J Radiat Oncol Biol Phys 83 (5): 1541-8, 2012.
- Merchant TE, Boop FA, Kun LE, et al.: A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 71 (1): 87-97, 2008.
- Kano H, Niranjan A, Kondziolka D, et al.: Outcome predictors for intracranial ependymoma radiosurgery. Neurosurgery 64 (2): 279-87; discussion 287-8, 2009.
- Eaton BR, Chowdhry V, Weaver K, et al.: Use of proton therapy for re-irradiation in pediatric intracranial ependymoma. Radiother Oncol 116 (2): 301-8, 2015.
Changes to This Summary (06 / 13 / 2019)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® – NCI’s Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood ependymoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Ependymoma Treatment are:
- Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Roger J. Packer, MD (Children’s National Health System)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Ependymoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-ependymoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389373]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Last Revised: 2019-06-13
Topic Contents
- General Information About Childhood Ependymoma
- Histopathologic Classification of Childhood Ependymal Tumors
- Stage Information for Childhood Ependymoma
- Treatment Option Overview for Childhood Ependymoma
- Treatment of Newly Diagnosed Childhood Subependymoma
- Treatment of Newly Diagnosed Childhood Myxopapillary Ependymoma
- Treatment of Newly Diagnosed Childhood Ependymoma, Anaplastic Ependymoma, or RELA Fusion–Positive Ependymoma
- Treatment of Recurrent Childhood Ependymoma
- Changes to This Summary (06 / 13 / 2019)
- About This PDQ Summary
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.

