Oral Cavity, Pharyngeal, and Laryngeal Cancer Prevention (PDQ®): Prevention – Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
Overview
Oral cavity, pharyngeal, and laryngeal cancers may be referred to as head and neck squamous cell cancers. Head and neck squamous cell cancers most commonly arise from the mucosal surfaces lining the oral cavity, pharynx, and larynx. Pharyngeal squamous cell cancers are further categorized into nasopharyngeal, oropharyngeal, and hypopharyngeal cancers on the basis of anatomical landmarks (refer to the PDQ summaries on Laryngeal Cancer Treatment (Adult), Lip and Oral Cavity Cancer Treatment (Adult), Nasopharyngeal Cancer Treatment (Adult), and Oropharyngeal Cancer Treatment (Adult) for more information). Figure 1 shows the anatomy of the pharynx.
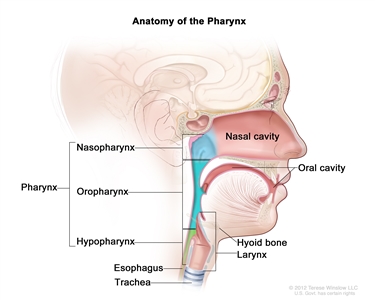
Figure 1. Anatomy of the pharynx.
Who Is at Risk?
Head and neck squamous cell cancers have common risk factors. People who use tobacco in any of the commonly available forms (cigarettes, cigars, pipes, and smokeless tobacco) or have a high alcohol intake are at elevated risk of oral cavity, oropharyngeal, hypopharyngeal, and laryngeal cancers; they are at particularly high risk if they use both tobacco and alcohol. Individuals who chew betel quid (whether mixed with tobacco or not) are also at high risk of cancer of the oral cavity and oropharynx.[1,2] Individuals who have a personal history of cancer of the head and neck region also are at elevated risk of a future primary cancer of the oral cavity or oropharynx.[3] Human papillomavirus (HPV) 16 is a sufficient, but not necessary, cause of oral, tongue, and oropharyngeal cancers.[4] Risk factors for nasopharyngeal cancer include heavy alcohol intake, family history, Chinese and other Asian ancestry, and Epstein-Barr virus (EBV)–persistent infection; smoking is not a risk factor for nasopharyngeal cancer.[5,6]
Note: Separate PDQ summaries on Oral Cavity, Pharyngeal, and Laryngeal Cancer Screening and Cigarette Smoking: Health Risks and How to Quit are also available.
Factors With Adequate Evidence of an Increased Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Tobacco use
Based on solid evidence from numerous observational studies, tobacco use increases the risk of cancers of the oral cavity and oropharynx, hypopharynx, and larynx.[7,8,9]
Magnitude of Effect: Large. Risk for current smokers is about tenfold that of never-smokers, and is dose related. Most cancers of the oral cavity, oropharynx, hypopharynx, and larynx are attributable to the use of tobacco products.
| Study Design: Numerous observational case-control and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Alcohol use
Based on solid evidence, alcohol use is a risk factor for the development of head and neck cancers. Its effects are independent of those of tobacco use.[10,11,12,13]
Magnitude of Effect: Lower than the risk associated with tobacco use, but the risk is approximately fivefold for people who drink five or more alcoholic beverages per day compared with nondrinkers, and is dose related.
| Study Design: Case-control and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Tobacco and alcohol use
The risk of oral cavity, oropharyngeal, hypopharyngeal, and laryngeal cancers is highest in people who consume large amounts of both alcohol and tobacco. When both risk factors are present, the risk of cancer is greater than a simple multiplicative effect of the two individual risks.[11,14]
Magnitude of Effect: About two to three times greater for oral cavity and oropharyngeal cancers than the simple multiplicative effect, with risks for persons who both smoke and drink heavily approximately 35-fold that of persons who both never smoke and never drink.[11,14,15]
| Study Design: Case-control and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Betel-quid chewing
Based on solid evidence, chewing betel quid alone or with added tobacco (gutka) increases the risk of both oral cavity and oropharyngeal cancers.[1,2] Of the three primary components of betel quid (betel leaf, areca nut, and lime), the areca nut is the only one considered to be carcinogenic when chewed.
Magnitude of Effect: Relative risks for oral cavity cancer are high and typically stronger for gutka than for betel quid alone. Both products appear to confer a modest yet statistically significant increase in risk of oropharyngeal cancer.[2]
| Study Design: Ecologic, case-control, and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Human papillomavirus (HPV) infection
Based on solid evidence, HPV 16 infection causes oropharyngeal cancer.[4,16] Other high-risk HPV subtypes, including HPV 18, have been found in a small percentage of oropharyngeal cancers.[17,18]
Tobacco and alcohol use does not appear to be a major risk factor among people with evidence of HPV 16 L1 seropositivity or oral HPV 16 infection.[17]
Magnitude of Effect: Large. Oral infection with HPV 16 confers about a 15-fold increase in risk of oropharyngeal cancer relative to individuals without oral HPV 16 infection.
| Study Design: Case-control and cohort studies, including one conducted using data collected prospectively (nested case-control study). |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Epstein Barr virus (EBV) infection
Based on solid evidence, EBV infection causes nasopharyngeal cancer in high-incidence areas.[6] Collective evidence includes numerous case-control studies and cohort studies that show a higher proportion of patients with nasopharyngeal cancer who have anti-EBV antibodies than controls and that seropositive status precedes tumor diagnosis.[19,20] Recent studies have also found circulating cell-free EBV DNA in patients with nasopharyngeal cancer but not in controls.[21] EBV alone is not a sufficient cause because 90% of adults worldwide are infected with the virus, but only a small proportion develop nasopharyngeal cancer.[6]
Magnitude of Effect: Large. Infection with EBV confers an increase in risk that is more than 33-fold higher than that in individuals without EBV infection.
| Study Design: Numerous case-control and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Interventions With Adequate Evidence of a Decreased Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Tobacco cessation
Based on solid evidence, cessation of exposure to tobacco (e.g., cigarettes, pipes, cigars, and smokeless tobacco) leads to a decrease in the risk of oral cavity, oropharyngeal, hypopharyngeal, and laryngeal cancers 20 years or longer after cessation.[22]
Magnitude of Effect: Decreased risk, moderate to large magnitude.
| Study Design: Case-control and cohort studies. |
| Internal Validity: Good. |
| Consistency: Good. |
| External Validity: Good. |
Interventions With Inadequate Evidence of a Reduced Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Cessation of alcohol consumption
Based on fair evidence, cessation of alcohol consumption leads to a decrease in oral cavity cancer, 20 years or more after cessation.[22] There is no evidence of a decrease in laryngeal cancer with alcohol cessation.[15]
Magnitude of Effect: Decreased risk, small to moderate magnitude.
| Study Design: Case-control studies. |
| Internal Validity: Fair. |
| Consistency: Fair. |
| External Validity: Fair. |
Vaccination against HPV 16 and the other high-risk subtypes
Vaccination against HPV 16 and 18 has been shown to prevent approximately 90% of oral HPV 16/18 infections within 4 years of vaccination.[23] However, no data are available to assess whether vaccination at any age will lead to reduced risk of oropharyngeal cancer at current typical ages of diagnosis.[24]
| Study Design: No studies available. |
| Internal Validity: Not applicable (N/A). |
| Consistency: N/A. |
| External Validity: N/A. |
References:
- Song H, Wan Y, Xu YY: Betel quid chewing without tobacco: a meta-analysis of carcinogenic and precarcinogenic effects. Asia Pac J Public Health 27 (2): NP47-57, 2015.
- Guha N, Warnakulasuriya S, Vlaanderen J, et al.: Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer 135 (6): 1433-43, 2014.
- Atienza JA, Dasanu CA: Incidence of second primary malignancies in patients with treated head and neck cancer: a comprehensive review of literature. Curr Med Res Opin 28 (12): 1899-909, 2012.
- Kreimer AR, Johansson M, Waterboer T, et al.: Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 31 (21): 2708-15, 2013.
- Cao SM, Simons MJ, Qian CN: The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30 (2): 114-9, 2011.
- Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15 (10): 1765-77, 2006.
- The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, Ga: U.S. Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004. Also available online. Last accessed March 6, 2019.
- National Cancer Institute: Cigars: Health Effects and Trends. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, [1998]. Smoking and Tobacco Control Monograph 9. Available online. Last accessed April 3, 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum 89: 1-592, 2007.
- Lubin JH, Muscat J, Gaudet MM, et al.: An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case-control studies. Cancer Causes Control 22 (9): 1217-31, 2011.
- Blot WJ, McLaughlin JK, Winn DM, et al.: Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 48 (11): 3282-7, 1988.
- Altieri A, Bosetti C, Gallus S, et al.: Wine, beer and spirits and risk of oral and pharyngeal cancer: a case-control study from Italy and Switzerland. Oral Oncol 40 (9): 904-9, 2004.
- Talamini R, La Vecchia C, Levi F, et al.: Cancer of the oral cavity and pharynx in nonsmokers who drink alcohol and in nondrinkers who smoke tobacco. J Natl Cancer Inst 90 (24): 1901-3, 1998.
- Hashibe M, Sturgis EM: Epidemiology of oral-cavity and oropharyngeal carcinomas: controlling a tobacco epidemic while a human papillomavirus epidemic emerges. Otolaryngol Clin North Am 46 (4): 507-20, 2013.
- Talamini R, Bosetti C, La Vecchia C, et al.: Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control 13 (10): 957-64, 2002.
- Hobbs CG, Sterne JA, Bailey M, et al.: Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 31 (4): 259-66, 2006.
- D’Souza G, Kreimer AR, Viscidi R, et al.: Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356 (19): 1944-56, 2007.
- Steinau M, Saraiya M, Goodman MT, et al.: Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis 20 (5): 822-8, 2014.
- Henle W, Henle G, Ho HC, et al.: Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst 44 (1): 225-31, 1970.
- Chien YC, Chen JY, Liu MY, et al.: Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345 (26): 1877-82, 2001.
- Lin JC, Wang WY, Chen KY, et al.: Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 350 (24): 2461-70, 2004.
- Marron M, Boffetta P, Zhang ZF, et al.: Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol 39 (1): 182-96, 2010.
- Herrero R, Quint W, Hildesheim A, et al.: Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 8 (7): e68329, 2013.
- Chaturvedi AK, Graubard BI, Broutian T, et al.: Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol 36 (3): 262-267, 2018.
Description of the Evidence
Incidence and Mortality
Oral cavity and oropharynx cancers
From 2010 to 2014, the estimated age-adjusted incidence of cancer of the oral cavity and oropharynx in the United States was 11.2 cases per 100,000 persons per year. The estimated mortality rate for the same years was 2.5 per 100,000 persons per year. U.S. incidence and mortality rates are both about 2.6 times higher in men than in women.[1] It is estimated that there will be 53,000 new cases of oral cavity and oropharynx cancer diagnosed in the United States in 2019 and 10,860 deaths due to this disease.[2] Rates of oral cavity cancer vary greatly across the world, primarily because of differences in alcohol use, tobacco use, and betel-quid chewing and the products chewed.
Although localized cancers of the oral cavity and oropharynx have an excellent anticipated 5-year survival rate of about 84%, only 29% of these cancer cases are diagnosed at this stage. The 5-year survival rate for patients with cancers of the oral cavity and oropharynx combined is only 65%;[2] however, the 5-year survival rate is much lower in African Americans (48%) than in whites (66%).[2]
Laryngeal cancer
Laryngeal cancers are less common with an annual incidence of 3 cases per 100,000 persons.[2] It is estimated that 12,410 new cases will be diagnosed in 2019, and an estimated 3,760 individuals will die from the disease.[2] The 5-year survival rate for laryngeal cancer is 61%.[2] New cases of laryngeal cancer have been falling an average of 2.4% per year over the last 10 years. This has been attributed to a reduction in cigarette smoking.
Hypopharyngeal cancer
Hypopharyngeal cancers are rare with approximately 2,500 new cases diagnosed in the United States each year and an annual incidence of 0.7 cases per 100,000 persons.[2,3] The 5-year survival rate for hypopharyngeal cancer is 26%.[3] New cases have been falling an average of 2% per year over the last 20 years.[3] This has been attributed to a reduction in cigarette smoking.
Nasopharyngeal cancer
Nasopharyngeal cancers are rare in the United States with an annual incidence rate of 0.7 cases per 100,000 persons.[4] However, there are marked geographic differences with an overall incidence in China that is 40- to 380-fold higher compared with the United States.[5] There are elevated rates of nasopharyngeal cancers in the Cantonese population of southern China (including Hong Kong), and intermediate rates are observed in several indigenous populations in Southeast Asia, and in natives of the Arctic region, North Africa, the Middle East, and southern China. First-generation Chinese immigrants to the United States maintain a high incidence rate, while their descendants born in the United States show a decreased incidence. The 5-year survival rate for keratinizing squamous cell carcinoma, the most common subtype of nasopharyngeal cancer in the United States, is 46%.[6]
Factors With Adequate Evidence of an Increased Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Tobacco use
Tobacco use is implicated in most cases of oral cavity, oropharyngeal, hypopharyngeal, and laryngeal cancers.[2] All forms of tobacco use (cigarettes, pipes, cigars, snuff, chewing tobacco, gutka [betel quid with tobacco added], and other smoked and smokeless products) increase the risk of these cancers.[7,8] Epidemiologic studies consistently demonstrate that cigarette smokers have a higher incidence of mortality from head and neck squamous cell cancers compared with lifetime nonsmokers, and there is general consensus that the relationship is causal. Among current smokers who smoke only cigarettes, the relative risk (RR) of cancer of the oral cavity or oropharynx was observed in a large cohort study to be approximately tenfold greater in men and fivefold greater in women compared with lifetime nonsmokers.[8] However, other epidemiologic studies have observed smaller and larger increases in risk, with some variation by anatomic location. Gutka chewing is prevalent in many countries in south and southeast Asia, including China and India, and is an important risk factor for both oral cavity and oropharyngeal cancers.[7]
Alcohol use
Alcohol use is a major independent risk factor for the development of head and neck squamous cell cancer.[9] Most epidemiologic studies demonstrate an increase in risk with increasing drinks per day, with a more than fivefold to sixfold increase in risk for individuals who consume five or more drinks a day relative to nonconsumers.[10,11] Associations are observed in studies that control for confounding by smoking, as well as in studies of nonsmokers.[9] There is a suggestion that consumption of beer and hard liquor confers a greater risk than does wine consumption.[12]
Tobacco and alcohol use
Head and neck squamous cell cancer risk is highest in people who consume large amounts of both alcohol and tobacco.[10] When both risk factors are present, the risk of oral cavity and oropharyngeal cancer is typically about two to three times greater than a simple multiplicative or additive effect.[12] In a case-control study, individuals who consumed two or more packs of cigarettes and more than four alcoholic drinks per day had slightly more than a 35-fold increased risk of developing oral cavity or oropharyngeal cancer, relative to individuals who neither smoked nor drank.[12] In a case-control study of laryngeal cancer, combined alcohol and tobacco consumption showed a multiplicative effect (odds ratio [OR], 177) rather than an additive risk.[11]
Betel-quid chewing
Betel quid is composed of betel leaf, areca nut, and lime; gutka is betel quid with added tobacco. Both betel-quid and gutka chewing increase the risk of cancer of the oral cavity and oropharynx.[7,13] The carcinogenic component of chewed betel quid arises from the areca nut.[7]
RRs are typically stronger for gutka than for betel quid alone.[13] A meta-analysis of oral cavity cancer studies conducted on the Indian subcontinent calculated a statistically significant eightfold increase in risk for gutka chewing and a statistically significant twofold increase in risk for betel-quid chewing. A statistically significant tenfold increase in oral cavity cancer risk for betel-quid chewing was demonstrated by studies conducted in China or Taiwan. A meta-analysis of oropharyngeal cancer studies conducted on the Indian subcontinent calculated a statistically significant fourfold increase in risk for gutka chewing and a statistically significant twofold increase in risk for betel-quid chewing.[13] Studies of head and neck cancer (without specification of subsite) suggest that increases in risk are positively correlated with chewing frequency and duration.[7]
Human papillomavirus (HPV) infection
HPV 16 infection is a sufficient, but not necessary, cause of head and neck cancers, with a greater causal relationship with oropharyngeal cancer.[14,15] A meta-analysis of five case-control studies of HPV 16 positivity in either serum or tissue calculated an OR of 15.1 (95% confidence interval [CI], 6.8–33.7) for cancer of the tonsils, 4.3 (95% CI, 2.1–8.9) for other oropharyngeal sites, and 2.0 for both oral cavity (95% CI, 1.2–3.4) and larynx (95% CI, 1.0–4.2).[15] In a case-control study, the observed strong association of HPV 16 serologic status and oropharyngeal cancer did not vary at different levels of tobacco or alcohol use.[16] A recent national survey observed that men have a higher prevalence of oral HPV than women (11.5% vs. 3.2%), including high-risk HPV subtypes (7.3% men; 1.4% women).[17]
Other high-risk HPV subtypes, including HPV 18, have been found in a small percentage of oropharyngeal cancers.[16,18] Given its association with cervical cancer, HPV 18 is believed to increase oropharyngeal cancer risk as well.[18]
Epstein Barr virus (EBV) infection
Based on solid evidence, EBV infection causes nasopharyngeal cancer in high-incidence areas.[19] One of the first studies to show an association was a cohort study that found higher anti-EBV titers in 84% of the 235 East African and Chinese patients with nasopharyngeal carcinoma.[20] The same study found higher anti-EBV titers with higher-stage tumor, and a case-control component of the study revealed that high anti-EBV titers were six times more likely in patients with nasopharyngeal carcinoma than in patients with head and neck cancers at other sites.
Other studies show elevation in both IgG and IgA antibody titers to EBV viral capsid antigen and other latent viral antigens, which precede tumor development by several years and are correlated with tumor burden, remission, and recurrence.[20,21] A large cohort study with 9,699 men measured both IgA antibodies against EBV capsid antigen and neutralizing antibodies against EBV-specific DNase and followed them for a later diagnosis of nasopharyngeal cancer.[21] The RR of nasopharyngeal carcinoma was 32.8 for individuals with both antibody markers (95% CI, 7.3–147.2; P < .001), and 4.0 for individuals with one marker (95% CI, 1.6–10.2; P = .003), compared with individuals with neither marker. There was a temporal relationship in that the difference in cumulative incidence between seropositive and seronegative patients increased with a longer duration of follow-up. Another study found circulating cell-free EBV DNA in 95% of patients with advanced nasopharyngeal cancer but not in controls or cured patients.[22]
EBV infection is subclinical and occurs early in childhood. EBV alone is not a sufficient cause because 90% of adults worldwide are infected with the virus, but only a small proportion of them develop nasopharyngeal cancer.[20] The pathogenesis is thought to involve the virus establishing latency in epithelial cells that have already undergone premalignant genetic changes.
Interventions With Adequate Evidence of a Decreased Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Tobacco cessation
The cessation of cigarette smoking is associated with an approximately 50% reduction in risk of developing oral cavity and oropharyngeal cancer within 5 to 9 years [23] and a return to a cancer risk comparable to that of never-smokers within 20 years.[23]
A case-control study matched 527 individuals with laryngeal cancer to 1,297 hospital controls and found that compared with never-smokers, smokers had a 20-fold increased risk of cancer and ex-smokers had a 7-fold increased risk.[11]
Dentists can participate in the full scope of pharmacological and behavioral interventions for smoking cessation.[24] A study has shown that only 25% of tobacco users report receiving advice to quit tobacco use from their dentists,[25] a proportion less than tobacco users who received such advice from their physicians.
Interventions With Inadequate Evidence of a Reduced Risk of Oral Cavity, Pharyngeal, and Laryngeal Cancers
Cessation of alcohol consumption
Because alcohol is associated with oral cavity and oropharyngeal cancer in a dose-dependent fashion,[12,26,27,28] it is believed that cessation or avoidance of alcohol use would result in reduced incidence. However, the evidence for reduced oral cavity and oropharyngeal cancer among people who have stopped consuming alcohol is inadequate.[23] Most studies suggest that the risk of oral cavity cancer decreases as time from cessation increases; one meta-analysis of eight studies observed a statistically significant 35% reduction (95% CI, 0.26%–0.78%), relative to current drinkers, for those who ceased consumption 20 years or more ago. Data for oropharyngeal cancer alone are not available, but studies that examine oropharyngeal cancer in conjunction with at least one other pharyngeal cancer typically have demonstrated a smaller risk reduction than for oral cavity cancer.[23] A case-control study of risk factors for laryngeal cancer found that alcohol cessation had no favorable effect on cancer risk.[11]
Vaccination against HPV 16 and other high-risk subtypes
Vaccination against HPV 16 and 18 has been shown to prevent approximately 90% of oral HPV 16/18 infections within 4 years of vaccination.[29,30] Given the relatively recent adoption of vaccination and the age at which individuals are vaccinated, there is not yet evidence that vaccination at a young age will lead to a substantially reduced risk of HPV-associated oropharyngeal cancer later in life. In addition, no data are available to examine whether incidence or mortality would be reduced if vaccination occurred at an age closer to that at which oropharyngeal cancers tend to present.
References:
- Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review (CSR) 1975-2014. Bethesda, Md: National Cancer Institute. Also available online. Last accessed February 8, 2019.
- American Cancer Society: Cancer Facts and Figures 2019. Atlanta, Ga: American Cancer Society, 2019. Available online. Last accessed January 23, 2019.
- Kuo P, Chen MM, Decker RH, et al.: Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope 124 (9): 2064-9, 2014.
- Richey LM, Olshan AF, George J, et al.: Incidence and survival rates for young blacks with nasopharyngeal carcinoma in the United States. Arch Otolaryngol Head Neck Surg 132 (10): 1035-40, 2006.
- Cao SM, Simons MJ, Qian CN: The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30 (2): 114-9, 2011.
- Ou SH, Zell JA, Ziogas A, et al.: Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol 18 (1): 29-35, 2007.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum 85: 1-334, 2004.
- Cancer. In: The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, Ga: U.S. Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004, pp 35-360. Also available online. Last accessed March 29, 2018.
- Goldstein BY, Chang SC, Hashibe M, et al.: Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: an update. Eur J Cancer Prev 19 (6): 431-65, 2010.
- Huber MA, Tantiwongkosi B: Oral and oropharyngeal cancer. Med Clin North Am 98 (6): 1299-321, 2014.
- Talamini R, Bosetti C, La Vecchia C, et al.: Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control 13 (10): 957-64, 2002.
- Blot WJ, McLaughlin JK, Winn DM, et al.: Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 48 (11): 3282-7, 1988.
- Guha N, Warnakulasuriya S, Vlaanderen J, et al.: Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer 135 (6): 1433-43, 2014.
- Kreimer AR, Johansson M, Waterboer T, et al.: Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 31 (21): 2708-15, 2013.
- Hobbs CG, Sterne JA, Bailey M, et al.: Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol 31 (4): 259-66, 2006.
- D’Souza G, Kreimer AR, Viscidi R, et al.: Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356 (19): 1944-56, 2007.
- Sonawane K, Suk R, Chiao EY, et al.: Oral Human Papillomavirus Infection: Differences in Prevalence Between Sexes and Concordance With Genital Human Papillomavirus Infection, NHANES 2011 to 2014. Ann Intern Med 167 (10): 714-724, 2017.
- Steinau M, Saraiya M, Goodman MT, et al.: Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg Infect Dis 20 (5): 822-8, 2014.
- Chang ET, Adami HO: The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15 (10): 1765-77, 2006.
- Henle W, Henle G, Ho HC, et al.: Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst 44 (1): 225-31, 1970.
- Chien YC, Chen JY, Liu MY, et al.: Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345 (26): 1877-82, 2001.
- Lin JC, Wang WY, Chen KY, et al.: Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 350 (24): 2461-70, 2004.
- Marron M, Boffetta P, Zhang ZF, et al.: Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol 39 (1): 182-96, 2010.
- Mecklenburg RE, Christen AG, et al.: How to Help Your Patients Stop Using Tobacco: a National Cancer Institute Manual for the Oral Health Team. Bethesda, Md: National Institutes of Health, National Cancer Institute, 1993.
- Martin LM, Bouquot JE, Wingo PA, et al.: Cancer prevention in the dental practice: oral cancer screening and tobacco cessation advice. J Public Health Dent 56 (6): 336-40, 1996 Fall.
- Macfarlane GJ, Zheng T, Marshall JR, et al.: Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol 31B (3): 181-7, 1995.
- La Vecchia C, Tavani A, Franceschi S, et al.: Epidemiology and prevention of oral cancer. Oral Oncol 33 (5): 302-12, 1997.
- Bagnardi V, Blangiardo M, La Vecchia C, et al.: Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 25 (4): 263-70, 2001.
- Herrero R, Quint W, Hildesheim A, et al.: Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 8 (7): e68329, 2013.
- Chaturvedi AK, Graubard BI, Broutian T, et al.: Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol 36 (3): 262-267, 2018.
Changes to This Summary (04 / 04 / 2019)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Description of the Evidence
Updated statistics with estimated new cases and deaths for oral cavity and oropharynx cancers for 2019 (cited American Cancer Society as reference 2).
Revised text to state that although localized cancers of the oral cavity and oropharynx have an anticipated 5-year survival rate of about 84%, only 29% of these cancer cases are diagnosed at this stage; the 5-year survival rate for patients with these cancers combined is only 65%, however, the 5-year survival rate is much lower in African Americans than in whites.
Updated statistics with estimated new cases and deaths for laryngeal cancer for 2019.
This summary is written and maintained by the PDQ Screening and Prevention Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® – NCI’s Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about oral cavity, pharyngeal, and laryngeal cancer prevention. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Screening and Prevention Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Oral Cavity, Pharyngeal, and Laryngeal Cancer Prevention. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/head-and-neck/hp/oral-prevention-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389416]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Last Revised: 2019-04-04
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.

