Oral Cavity, Pharyngeal, and Laryngeal Cancer Screening (PDQ®): Screening – Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
Overview
Oral cavity, pharyngeal, and laryngeal cancers may be referred to as head and neck squamous cell cancers. Head and neck squamous cell cancers most commonly arise from the mucosal surfaces lining the oral cavity, pharynx, and larynx. Pharyngeal squamous cell cancers are further categorized into nasopharyngeal, oropharyngeal, and hypopharyngeal cancers on the basis of anatomical landmarks. Figure 1 shows the anatomy of the pharynx.
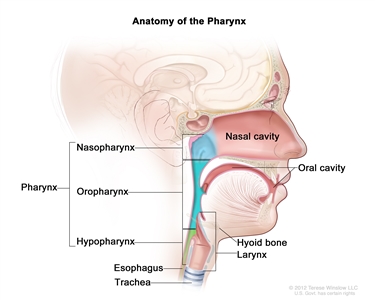
Figure 1. Anatomy of the pharynx.
Note: Separate PDQ summaries on Oral Cavity, Pharyngeal, and Laryngeal Cancer Prevention and Lip and Oral Cavity Cancer Treatment (Adult) are also available.
Benefits
There is inadequate evidence to establish whether screening would result in a decrease in mortality from head and neck squamous cell cancers.
Magnitude of Effect: No evidence of benefit or harm.
| Study Design: Evidence obtained from one randomized controlled trial and observational studies. |
| Internal Validity: Poor. |
| Consistency: Not applicable (N/A). |
| External Validity: Poor. |
Harms
Harms have not been systematically studied and cannot be quantified on the basis of the literature. However, there are some unavoidable harms that would be associated with routine screening, including:
- Unnecessary treatment associated with overdiagnosis.
- Psychologic consequences of false-positive tests.
- Misdiagnosis due to variability in assessment of biopsies.
Magnitude of Effect: Unknown.
| Study Design: Observational studies. |
| Internal Validity: Poor. |
| Consistency: N/A. |
| External Validity: Poor. |
Description of the Evidence
Incidence and Mortality
An estimated 53,000 new cases of oral cavity and oropharynx cancers will be diagnosed in the United States in 2019, and an estimated 10,860 people will die of these diseases.[1] The overall annual incidence in the United States is about 11 cases per 100,000 men and women; the incidence rate is highest in individuals aged 55 to 64 years.[2]
From 2006 to 2015, incidence rates increased by 1.2% per year among whites and declined by 2.3% per year among African Americans.[1] The incidence has been increasing for oral cavity and oropharyngeal cancers related to human papillomavirus (HPV) infection. About 60% of oral/pharyngeal cancers are moderately advanced (regional stage) or metastatic at the time of diagnosis.[2] The 5-year survival rate is 65%.[1]
The estimated annual worldwide number of incidents of oral cavity and oropharyngeal cancers is about 275,000, with an approximate 20-fold variation geographically.[3] South and Southeast Asia (India, Sri Lanka, Pakistan, and Bangladesh), France, and Brazil have particularly high rates. In most countries, men have higher rates of oral cancer than women (caused by tobacco use) and higher rates of lip cancer (caused by sunlight exposure from outdoor occupations).[3]
Laryngeal cancers are less common, with an annual incidence of 3 cases per 100,000 persons. It is estimated that 12,410 new cases will be diagnosed in the United States in 2019, and an estimated 3,760 people will die of the disease. The 5-year survival rate for laryngeal cancer is 61%.[1] New cases of laryngeal cancer have been falling, on average, 2.4% per year in the last 10 years. This decline has been attributed to a reduction in cigarette smoking.
Hypopharyngeal cancers are rare, with approximately 2,500 new cases diagnosed in the United States each year and an annual incidence of 0.7 cases per 100,000 persons.[4,5] The 5-year survival rate for hypopharyngeal cancer is 26%.[5] New cases have been falling, on average, 2% per year in the last 20 years.[5] This decline has been attributed to a reduction in cigarette smoking.
Nasopharyngeal cancers are rare in the United States, with an annual incidence rate of 0.7 cases per 100,000 persons.[6] However, there are marked geographic differences, with an overall incidence in China that is 40- to 380-fold higher than that in the United States.[6] There are elevated rates of nasopharyngeal cancers in the Cantonese population of southern China (including Hong Kong), and intermediate rates are observed in several indigenous populations in Southeast Asia and in natives of the Arctic region, North Africa, and the Middle East. First-generation Chinese immigrants to the United States maintain a high incidence rate, while their descendants born in the United States show a decreased incidence. The 5-year survival rate for keratinizing squamous cell carcinoma, the most common subtype of nasopharyngeal cancer in the United States, is 46%.[7]
Risk Factors
The primary risk factors for oral cavity, oropharynx, hypopharynx, and laryngeal cancers in American men and women are tobacco (including smokeless tobacco) use and alcohol use. Infection with HPV-16 has been associated with an excess risk of developing squamous cell carcinoma of the oral tongue and oropharynx.[8]
Risk factors for nasopharyngeal cancer include heavy alcohol intake (but not smoking), family history, Chinese (or Asian) ancestry, and Epstein-Barr virus (EBV) persistent infection.[9]
Refer to the PDQ summary on Oral Cavity, Pharyngeal, and Laryngeal Cancer Prevention for a complete description of factors associated with an increased or decreased risk of head and neck squamous cell cancers.
Evidence of Benefit Associated With Screening
No population-based screening programs for head and neck squamous cell cancers have been implemented in developed countries, although opportunistic screening or screening as part of a periodic health examination has been advocated for the oral cavity, which is the only site accessible without endoscopy.[10,11]
Screening for oral cavity cancers
There are different methods of screening for oral cavity cancers. Oral cavity cancers occur in a region of the body that is generally accessible to physical examination by the patient, the dentist, and the physician; and visual examination is the most common method used to detect visible lesions. Other methods have been used to augment clinical detection of oral lesions and include toluidine blue, brush biopsy, and fluorescence staining.
An inspection of the oral cavity is often part of a physical examination in a dentist’s or physician’s office. It has been pointed out that high-risk individuals visit their medical doctors more frequently than they visit their dentists. Although physicians are more likely to provide risk-factor counseling (such as tobacco cessation), they are less likely than dentists to perform an oral cancer examination.[12] Overall, only a fraction (~20%) of Americans receive an oral cancer examination. Black patients, Hispanic patients, and those who have a lower level of education are less likely to have such an examination, perhaps because they lack access to medical care.[12] An oral examination often includes looking for leukoplakia and erythroplakia lesions, which can progress to cancer.[13,14] One study has shown that direct fluorescence visualization (using a simple hand-held device in the operating room) could identify subclinical high-risk fields with cancerous or precancerous changes extending up to 25 mm beyond the primary tumor in 19 of 20 patients undergoing oral surgery for invasive or in situ squamous cell tumors.[15] However, this finding has not yet been tested in a screening setting. Data suggest that molecular markers may be useful in the prognosis of these premalignant oral lesions.[16]
The routine examination of asymptomatic and symptomatic patients can lead to detection of earlier-stage cancers and premalignant lesions. There is no definitive evidence, however, to show that this screening can reduce oral cancer mortality, and there are no randomized controlled trials (RCTs) in any Western or other low-risk populations.[14,17,18,19,20]
In a single RCT of screening versus usual care, 13 geographic clusters in the Trivandrum district of Kerala, India, were randomly assigned to receive systematic oral visual screening by trained health workers (seven screened clusters, six control clusters) every 3 years for four screening rounds during the period 1996 to 2008. During a 15-year follow-up period, there were 138 deaths from oral cancer in the screening group, with a cause-specific mortality rate of 15.4 per 100,000 person-years, and 154 deaths in the control group, with a mortality rate of 17.1 per 100,000 person-years (relative risk [RR], 0.88; 95% confidence interval [CI], 0.69–1.12). In a subset analysis restricted to tobacco or alcohol users, the mortality rates were 30 and 39 per 100,000 person-years, respectively (RR, 0.76; 95% CI, 0.60–0.97). There was no apparent adjustment of the CIs for the cluster design. In another subgroup analysis, mortality hazard ratios were calculated for groups defined by number of times screened, but the inappropriate comparison in each case was to the control group of the whole study. No data on treatment of oral cancers were presented.[21,22,23,24]
Aside from the issues of generalizability to other populations and lack of an overall statistically significant result in cause-specific mortality, interpretation of the results is made difficult by serious lacks in methodologic detail about the randomization process, allocation concealment, adjustment for clustering effect, and information about treatment. The total number of clusters randomized was small, and there were different distributions of income and household possessions between the two study arms. Withdrawals and dropouts were not clearly described. In summary, the sole randomized trial does not provide solid evidence of a cause-specific mortality benefit associated with systematic oral cavity visual examination.
Techniques such as toluidine blue staining, brush biopsy/cytology, or fluorescence imaging as the primary screening tool or as an adjunct for screening have not been shown to have superior sensitivity and specificity for visual examination alone or to yield better health outcomes.[14,25] In an RCT conducted in Keelung County, Taiwan, 7,975 individuals at high risk of oral cancer due to cigarette smoking or betel-quid chewing were randomly assigned to receive a one-time oral cancer examination after gargling with toluidine blue or a blue placebo dye.[26] The positive test rates were 9.5% versus 8.3%, respectively (P = .047). The detection of premalignant lesions was not statistically different (rate ratio, 1.05; 95% CI, 0.74–1.41). The number of overall oral cancers diagnosed within the short follow-up period of 5 years was too small for valid comparison (six in each group).
The operating characteristics of the various techniques used as an adjunct to oral visual examination are not well established. A systematic literature review of toluidine blue, a variety of other visualization adjuncts, and cytopathology in the screening setting revealed a very broad range of reported sensitivities, specificities, and positive predictive values when biopsy confirmation was used as the gold standard outcome.[27] In part, this range of findings can be attributed to varying study populations, sample size and settings, and criteria for positive-clinical examinations and for scoring a biopsy as positive.
Screening for nasopharyngeal cancer
Serum EBV–associated antibodies and circulating cell-free EBV DNA testing have been used for nasopharyngeal cancer diagnosis and screening. In an observational study of 20,349 men aged 40 to 62 years, circulating cell-free EBV DNA testing was used to screen for nasopharyngeal cancer.[9,28] Of the 34 patients, 1.5% of participants were double-screen positive and had further workup, leading to a diagnosis of nasopharyngeal cancer. The EBV DNA test had a sensitivity of 97.1% (95% CI, 95.5%–98.7%) and specificity of 98.6% (95% CI, 98.6%–98.7%). Without a control group, the study compared stage of disease at diagnosis with a historical cohort and found a higher proportion of stage I and stage II disease (71% vs. 20%; P < .001) and superior 3-year progression-free survival in the screen-detected population. However, the survival benefit in the study may also be caused by lead-time bias.
Other screening programs in southern China use EBV-associated antibodies, but their effects are difficult to determine because of lack of controls for comparison of survival outcomes.[9,29,30] In summary, current screening studies for nasopharyngeal cancer do not provide solid evidence of a benefit associated with screening for nasopharyngeal cancer, especially in nonendemic regions such as the United States.
Evidence of Harm Associated With Screening
Harms associated with screening for head and neck squamous cell cancers are poorly studied in any quantifiable way.[20] However, there are some unavoidable harms that would be associated with routine screening, including:
- Unnecessary treatment of lesions that would not have progressed (overdiagnosis).
- Psychologic consequences of false-positive tests.[31]
An additional potential harm is misdiagnosis and resulting under- or overtreatment, given the subjective pathology judgments in the reading of biopsies of oral lesions. When 87 biopsy diagnoses of oral lesions were compared between 21 local pathologists and double-reading by two of three central pathologists in a multicenter study of patients with prior upper aerodigestive tract cancers, agreement was only fair to good (kappa-weighted statistic, 0.59; 95% CI, 0.45–0.72).[32] In a bivariate categorization of carcinoma in situ plus carcinoma versus less serious lesions, the agreement was poor, but with very wide CIs (kappa statistic, 0.39; 95% CI, -0.12 to -0.97). The investigators in the same study analyzed an agreement between the local and central pathologists on clinically normal tissue adjacent to 67 biopsied, clinically suspicious lesions. The agreement on clinically normal tissue was better than for visibly abnormal lesions, but still not in the excellent range (kappa-weighted statistic, 0.75; 95% CI, 0.64–0.86).[33]
References:
- American Cancer Society: Cancer Facts and Figures 2019. Atlanta, Ga: American Cancer Society, 2019. Available online. Last accessed January 23, 2019.
- Howlader N, Noone AM, Krapcho M: SEER Cancer Statistics Review (CSR) 1975-2013. Bethesda, Md: National Cancer Institute, 2015. Available online. Last accessed January 31, 2019.
- Warnakulasuriya S: Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45 (4-5): 309-16, 2009 Apr-May.
- American Cancer Society: Cancer Facts and Figures 2017. Atlanta, Ga: American Cancer Society, 2017. Available online. Last accessed February 7, 2019.
- Kuo P, Chen MM, Decker RH, et al.: Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope 124 (9): 2064-9, 2014.
- Richey LM, Olshan AF, George J, et al.: Incidence and survival rates for young blacks with nasopharyngeal carcinoma in the United States. Arch Otolaryngol Head Neck Surg 132 (10): 1035-40, 2006.
- Ou SH, Zell JA, Ziogas A, et al.: Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol 18 (1): 29-35, 2007.
- Mork J, Lie AK, Glattre E, et al.: Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med 344 (15): 1125-31, 2001.
- Cao SM, Simons MJ, Qian CN: The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 30 (2): 114-9, 2011.
- Opportunistic oral cancer screening: a management strategy for dental practice. BDA Occasional Paper 6: 1-36, 2000. Also available online. Last accessed April 3, 2019.
- Smith RA, Cokkinides V, Brooks D, et al.: Cancer screening in the United States, 2011: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 61 (1): 8-30, 2011 Jan-Feb.
- Kerr AR, Changrani JG, Gany FM, et al.: An academic dental center grapples with oral cancer disparities: current collaboration and future opportunities. J Dent Educ 68 (5): 531-41, 2004.
- Warnakulasuriya S, Johnson NW, van der Waal I: Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 36 (10): 575-80, 2007.
- Brocklehurst P, Kujan O, Glenny AM, et al.: Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev (11): CD004150, 2010.
- Poh CF, Zhang L, Anderson DW, et al.: Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res 12 (22): 6716-22, 2006.
- Poh CF, Zhang L, Lam WL, et al.: A high frequency of allelic loss in oral verrucous lesions may explain malignant risk. Lab Invest 81 (4): 629-34, 2001.
- Screening for oral cancer. In: Fisher M, Eckhart C, eds.: Guide to Clinical Preventive Services: an Assessment of the Effectiveness of 169 Interventions. Report of the U.S. Preventive Services Task Force. Baltimore, Md: Williams & Wilkins, 1989, pp 91-94.
- Antunes JL, Biazevic MG, de Araujo ME, et al.: Trends and spatial distribution of oral cancer mortality in São Paulo, Brazil, 1980-1998. Oral Oncol 37 (4): 345-50, 2001.
- U.S. Preventive Services Task Force: Screening for Oral Cancer: Recommendation Statement. Rockville, Md: U.S. Preventive Services Task Force, 2004. Available online. Last accessed April 3, 2019.
- Scattoloni J: Screening for Oral Cancer: Brief Evidence Update. Rockville, Md: U.S. Preventive Services Task Force, 2004. Available online. Last accessed April 3, 2019.
- Sankaranarayanan R, Mathew B, Jacob BJ, et al.: Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer 88 (3): 664-73, 2000.
- Ramadas K, Sankaranarayanan R, Jacob BJ, et al.: Interim results from a cluster randomized controlled oral cancer screening trial in Kerala, India. Oral Oncol 39 (6): 580-8, 2003.
- Sankaranarayanan R, Ramadas K, Thomas G, et al.: Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet 365 (9475): 1927-33, 2005 Jun 4-10.
- Sankaranarayanan R, Ramadas K, Thara S, et al.: Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol 49 (4): 314-21, 2013.
- Lingen MW, Kalmar JR, Karrison T, et al.: Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 44 (1): 10-22, 2008.
- Su WW, Yen AM, Chiu SY, et al.: A community-based RCT for oral cancer screening with toluidine blue. J Dent Res 89 (9): 933-7, 2010.
- Patton LL, Epstein JB, Kerr AR: Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc 139 (7): 896-905; quiz 993-4, 2008.
- Chan KCA, Woo JKS, King A, et al.: Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N Engl J Med 377 (6): 513-522, 2017.
- Zeng Y, Zhang LG, Li HY, et al.: Serological mass survey for early detection of nasopharyngeal carcinoma in Wuzhou City, China. Int J Cancer 29 (2): 139-41, 1982.
- Zeng Y, Zhong JM, Li LY, et al.: Follow-up studies on Epstein-Barr virus IgA/VCA antibody-positive persons in Zangwu County, China. Intervirology 20 (4): 190-4, 1983.
- Speight PM, Zakrzewska J, Downer MC: Screening for oral cancer and precancer. Eur J Cancer B Oral Oncol 28B (1): 45-8, 1992.
- Fischer DJ, Epstein JB, Morton TH, et al.: Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant lesions. J Oral Pathol Med 33 (2): 65-70, 2004.
- Fischer DJ, Epstein JB, Morton TH Jr, et al.: Reliability of histologic diagnosis of clinically normal intraoral tissue adjacent to clinically suspicious lesions in former upper aerodigestive tract cancer patients. Oral Oncol 41 (5): 489-96, 2005.
Changes to This Summary (04 / 04 / 2019)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Description of the Evidence
Updated statistics with estimated new cases and deaths for oral cavity and oropharynx cancers for 2019 (cited American Cancer Society as reference 1).
Revised text to state that from 2006 to 2015, incidence rates increased by 1.2% per year among whites and declined by 2.3% per year among African Americans.
Updated statistics with estimated new cases and deaths for laryngeal cancer for 2019.
This summary is written and maintained by the PDQ Screening and Prevention Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® – NCI’s Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about oral cavity, pharyngeal, and laryngeal cancer screening. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website’s Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Screening and Prevention Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Oral Cavity, Pharyngeal, and Laryngeal Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/head-and-neck/hp/oral-screening-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389219]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Last Revised: 2019-04-04
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.

